A critical role for endocytosis in Wnt signaling
- PMID: 16824228
- PMCID: PMC1534015
- DOI: 10.1186/1471-2121-7-28
A critical role for endocytosis in Wnt signaling
Abstract
Background: The Wnt signaling pathway regulates many processes during embryonic development, including axis specification, organogenesis, angiogenesis, and stem cell proliferation. Wnt signaling has also been implicated in a number of cancers, bone density maintenance, and neurological conditions during adulthood. While numerous Wnts, their cognate receptors of the Frizzled and Arrow/LRP5/6 families and downstream pathway components have been identified, little is known about the initial events occurring directly after receptor activation.
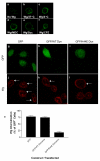
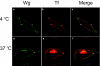
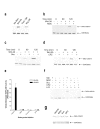
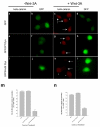
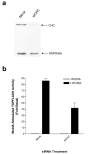
Results: We show here that Wnt proteins are rapidly endocytosed by a clathrin- and dynamin-mediated process. While endocytosis has traditionally been considered a principal mechanism for receptor down-regulation and termination of signaling pathways, we demonstrate that interfering with clathrin-mediated endocytosis actually blocks Wnt signaling at the level of beta-catenin accumulation and target gene expression.
Conclusion: A necessary component of Wnt signaling occurs in a subcellular compartment distinct from the plasma membrane. Moreover, as internalized Wnts transit partially through the transferrin recycling pathway, it is possible that a "signaling endosome" serves as a nexus for activated Wnt pathway components.
Figures
Similar articles
-
Wnt/Wingless signaling through beta-catenin requires the function of both LRP/Arrow and frizzled classes of receptors.BMC Cell Biol. 2003 May 2;4:4. doi: 10.1186/1471-2121-4-4. BMC Cell Biol. 2003. PMID: 12729465 Free PMC article.
-
Inhibition of endocytosis blocks Wnt signalling to beta-catenin by promoting dishevelled degradation.Acta Physiol (Oxf). 2007 May;190(1):55-61. doi: 10.1111/j.1365-201X.2007.01688.x. Acta Physiol (Oxf). 2007. PMID: 17428233
-
Drosophila Wnt/Fz pathways.Sci STKE. 2005 May 10;2005(283):cm5. doi: 10.1126/stke.2832005cm5. Sci STKE. 2005. PMID: 15886387
-
Molecular mechanism and physiological functions of clathrin-mediated endocytosis.Nat Rev Mol Cell Biol. 2011 Jul 22;12(8):517-33. doi: 10.1038/nrm3151. Nat Rev Mol Cell Biol. 2011. PMID: 21779028 Review.
-
Signalling and endocytosis: Wnt breaks down on back roads.Nat Cell Biol. 2001 Aug;3(8):E185-6. doi: 10.1038/35087126. Nat Cell Biol. 2001. PMID: 11483976 Review. No abstract available.
Cited by
-
Beta-arrestin is a necessary component of Wnt/beta-catenin signaling in vitro and in vivo.Proc Natl Acad Sci U S A. 2007 Apr 17;104(16):6690-5. doi: 10.1073/pnas.0611356104. Epub 2007 Apr 10. Proc Natl Acad Sci U S A. 2007. PMID: 17426148 Free PMC article.
-
R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/beta-catenin signaling.Proc Natl Acad Sci U S A. 2011 Jul 12;108(28):11452-7. doi: 10.1073/pnas.1106083108. Epub 2011 Jun 21. Proc Natl Acad Sci U S A. 2011. PMID: 21693646 Free PMC article.
-
Clathrin-dependent internalization, signaling, and metabolic processing of guanylyl cyclase/natriuretic peptide receptor-A.Mol Cell Biochem. 2018 Apr;441(1-2):135-150. doi: 10.1007/s11010-017-3180-0. Epub 2017 Sep 12. Mol Cell Biochem. 2018. PMID: 28900772 Free PMC article.
-
Temporal changes in plasma membrane lipid content induce endocytosis to regulate developmental epithelial-to-mesenchymal transition.Proc Natl Acad Sci U S A. 2022 Dec 20;119(51):e2212879119. doi: 10.1073/pnas.2212879119. Epub 2022 Dec 12. Proc Natl Acad Sci U S A. 2022. PMID: 36508654 Free PMC article.
-
Frizzled and LRP5/6 receptors for Wnt/β-catenin signaling.Cold Spring Harb Perspect Biol. 2012 Dec 1;4(12):a007880. doi: 10.1101/cshperspect.a007880. Cold Spring Harb Perspect Biol. 2012. PMID: 23209147 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

