Ca2+ refilling of the endoplasmic reticulum is largely preserved albeit reduced Ca2+ entry in endothelial cells
- PMID: 16824596
- PMCID: PMC4060231
- DOI: 10.1016/j.ceca.2006.05.001
Ca2+ refilling of the endoplasmic reticulum is largely preserved albeit reduced Ca2+ entry in endothelial cells
Abstract
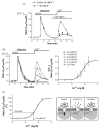
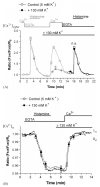
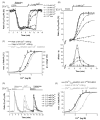
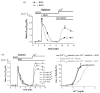
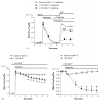
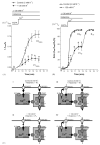
In this study the relationship between the efficiency of endoplasmic reticulum (ER) Ca2+ refilling and the extent of Ca2+ entry was investigated in endothelial cells. ER and mitochondrial Ca2+ concentration were measured using genetically encoded Ca2+ sensors, while the amount of entering Ca2+ was controlled by varying either the extracellular Ca2+ or the electrical driving force for Ca2+ by changing the plasma membrane potential. In the absence of an agonist, ER Ca2+ replenishment was fully accomplished even if the Ca2+ concentration applied was reduced from 2 to 0.5mM. A similar strong efficiency of ER Ca2+ refilling was obtained under condition of plasma membrane depolarization. However, in the presence of histamine, ER Ca2+ refilling depended on mitochondrial Ca2+ transport and was more susceptible to membrane depolarization. Store-operated Ca2+ entry (SOCE), was strongly reduced under low Ca2+ and depolarizing conditions but increased if ER Ca2+ uptake was blocked or if ER Ca2+ was released continuously by IP(3). A correlation of the kinetics of ER Ca2+refilling with cytosolic Ca2+ signals revealed that termination of SOCE is a rapid event that is not delayed compared to ER refilling. Our data indicate that ER refilling occurs in priority to, and independently from the cytosolic Ca2+ elevation upon Ca2+ entry and that this important process is widely achieved even under conditions of diminished Ca2+entry.
Figures

Similar articles
-
The role of mitochondria for Ca2+ refilling of the endoplasmic reticulum.J Biol Chem. 2005 Apr 1;280(13):12114-22. doi: 10.1074/jbc.M409353200. Epub 2005 Jan 19. J Biol Chem. 2005. PMID: 15659398
-
STIM1 knockdown reveals that store-operated Ca2+ channels located close to sarco/endoplasmic Ca2+ ATPases (SERCA) pumps silently refill the endoplasmic reticulum.J Biol Chem. 2007 Apr 13;282(15):11456-64. doi: 10.1074/jbc.M609551200. Epub 2007 Feb 5. J Biol Chem. 2007. PMID: 17283081
-
Mitochondria maintain maturation and secretion of lipoprotein lipase in the endoplasmic reticulum.Biochem J. 2006 May 15;396(1):173-82. doi: 10.1042/BJ20060099. Biochem J. 2006. PMID: 16466345 Free PMC article.
-
Remodelling of the endoplasmic reticulum during store-operated calcium entry.Biol Cell. 2011 Aug;103(8):365-80. doi: 10.1042/BC20100152. Biol Cell. 2011. PMID: 21736554 Review.
-
Privileged coupling between Ca(2+) entry through plasma membrane store-operated Ca(2+) channels and the endoplasmic reticulum Ca(2+) pump.Mol Cell Endocrinol. 2012 Apr 28;353(1-2):37-44. doi: 10.1016/j.mce.2011.08.021. Epub 2011 Aug 24. Mol Cell Endocrinol. 2012. PMID: 21878366 Review.
Cited by
-
The Molecular Heterogeneity of Store-Operated Ca2+ Entry in Vascular Endothelial Cells: The Different roles of Orai1 and TRPC1/TRPC4 Channels in the Transition from Ca2+-Selective to Non-Selective Cation Currents.Int J Mol Sci. 2023 Feb 7;24(4):3259. doi: 10.3390/ijms24043259. Int J Mol Sci. 2023. PMID: 36834672 Free PMC article. Review.
-
Enhanced spontaneous Ca2+ events in endothelial cells reflect signalling through myoendothelial gap junctions in pressurized mesenteric arteries.Cell Calcium. 2008 Aug;44(2):135-46. doi: 10.1016/j.ceca.2007.11.012. Epub 2008 Jan 11. Cell Calcium. 2008. PMID: 18191200 Free PMC article.
-
GPR55-dependent and -independent ion signalling in response to lysophosphatidylinositol in endothelial cells.Br J Pharmacol. 2010 Sep;161(2):308-20. doi: 10.1111/j.1476-5381.2010.00744.x. Br J Pharmacol. 2010. PMID: 20735417 Free PMC article.
-
PCV2 Triggers PK-15 Cell Apoptosis Through the PLC-IP3R-Ca2+ Signaling Pathway.Front Microbiol. 2021 Jun 15;12:674907. doi: 10.3389/fmicb.2021.674907. eCollection 2021. Front Microbiol. 2021. PMID: 34211446 Free PMC article.
-
The endocannabinoid N-arachidonoyl glycine (NAGly) inhibits store-operated Ca2+ entry by preventing STIM1-Orai1 interaction.J Cell Sci. 2013 Feb 15;126(Pt 4):879-88. doi: 10.1242/jcs.118075. Epub 2012 Dec 13. J Cell Sci. 2013. PMID: 23239024 Free PMC article.
References
-
- Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. - PubMed
-
- Parekh AB, Putney JWJ. Store-operated calcium channels. Physiol. Rev. 2005;85:757–810. - PubMed
-
- Putney JW., Jr. A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. - PubMed
-
- Putney JW., Jr. Capacitative calcium entry revisited. Cell Calcium. 1990;11:611–624. - PubMed
-
- Abad MF, Di Benedetto G, Magalhaes PJ, Filippin L, Pozzan T. Mitochondrial pH monitored by a new engineered green fluorescent protein mutant. J. Biol. Chem. 2004;279:11521–11529. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

