HIF-dependent hematopoietic factors regulate the development of the embryonic vasculature
- PMID: 16824955
- PMCID: PMC3145415
- DOI: 10.1016/j.devcel.2006.04.018
HIF-dependent hematopoietic factors regulate the development of the embryonic vasculature
Abstract
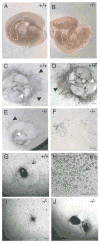
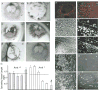
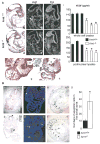
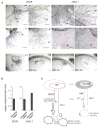
Hypoxia inducible factors (HIFs) regulate adaptive responses to changes in oxygen (O(2)) tension during embryogenesis, tissue ischemia, and tumorigenesis. Because HIF-deficient embryos exhibit a number of developmental defects, the precise role of HIF in early vascular morphogenesis has been uncertain. Using para-aortic splanchnopleural (P-Sp) explant cultures, we show that deletion of the HIF-beta subunit (ARNT) results in defective hematopoiesis and the inhibition of both vasculogenesis and angiogenesis. These defects are rescued upon the addition of wild-type Sca-1(+) hematopoietic cells or recombinant VEGF. Arnt(-/-) embryos exhibit reduced levels of VEGF protein and increased numbers of apoptotic hematopoietic cells. These results suggest that HIF coordinates early endothelial cell emergence and vessel development by promoting hematopoietic cell survival and paracrine growth factor production.
Figures

Similar articles
-
Hypoxia influences the vascular expansion and differentiation of embryonic stem cell cultures through the temporal expression of vascular endothelial growth factor receptors in an ARNT-dependent manner.Stem Cells. 2010 Apr;28(4):799-809. doi: 10.1002/stem.316. Stem Cells. 2010. PMID: 20135683 Free PMC article.
-
Hypoxia affects mesoderm and enhances hemangioblast specification during early development.Development. 2004 Sep;131(18):4623-34. doi: 10.1242/dev.01310. Development. 2004. PMID: 15342485
-
The aryl hydrocarbon receptor nuclear translocator is an essential regulator of murine hematopoietic stem cell viability.Blood. 2015 May 21;125(21):3263-72. doi: 10.1182/blood-2014-10-607267. Epub 2015 Apr 8. Blood. 2015. PMID: 25855602 Free PMC article.
-
Hypoxia-inducible factors in the kidney.Am J Physiol Renal Physiol. 2006 Aug;291(2):F271-81. doi: 10.1152/ajprenal.00071.2006. Epub 2006 Mar 22. Am J Physiol Renal Physiol. 2006. PMID: 16554418 Free PMC article. Review.
-
Hypoxia and HIFs in regulating the development of the hematopoietic system.Blood Cells Mol Dis. 2013 Dec;51(4):256-63. doi: 10.1016/j.bcmd.2013.08.005. Epub 2013 Oct 5. Blood Cells Mol Dis. 2013. PMID: 24103835 Free PMC article. Review.
Cited by
-
Cited2 gene controls pluripotency and cardiomyocyte differentiation of murine embryonic stem cells through Oct4 gene.J Biol Chem. 2012 Aug 17;287(34):29088-100. doi: 10.1074/jbc.M112.378034. Epub 2012 Jul 3. J Biol Chem. 2012. PMID: 22761414 Free PMC article.
-
HIF-1α may provide only short-term protection against ischemia-reperfusion injury in Sprague-Dawley myocardial cultures.Mol Clin Oncol. 2016 Apr;4(4):579-583. doi: 10.3892/mco.2016.764. Epub 2016 Feb 3. Mol Clin Oncol. 2016. PMID: 27073667 Free PMC article.
-
Hypoxia during incubation and its effects on broiler's embryonic development.Poult Sci. 2021 Mar;100(3):100951. doi: 10.1016/j.psj.2020.12.048. Epub 2021 Jan 5. Poult Sci. 2021. PMID: 33652530 Free PMC article. Review.
-
Inhibition of ARNT severely compromises endothelial cell viability and function in response to moderate hypoxia.Angiogenesis. 2012 Sep;15(3):409-20. doi: 10.1007/s10456-012-9269-x. Epub 2012 Apr 7. Angiogenesis. 2012. PMID: 22484908 Free PMC article.
-
Hypoxia-inducible factors and the response to hypoxic stress.Mol Cell. 2010 Oct 22;40(2):294-309. doi: 10.1016/j.molcel.2010.09.022. Mol Cell. 2010. PMID: 20965423 Free PMC article. Review.
References
-
- Arai P, Hirao A, Suda T. Regulation of hematopoietic stem cells by the niche. Trends Cardiovasc Med. 2005;15:75–79. - PubMed
-
- Brusselmans K, Bono F, Collen D, Herbert JM, Carmeliet P, Dewerchin M. A novel role for vascular endothelial growth factor as an autocrine survival factor for embryonic stem cells during hypoxia. J Biol Chem. 2005;280:3493–3499. - PubMed
-
- Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

