The inotropic effect of cardioactive glycosides in ventricular myocytes requires Na+-Ca2+ exchanger function
- PMID: 16825310
- PMCID: PMC1995692
- DOI: 10.1113/jphysiol.2006.111252
The inotropic effect of cardioactive glycosides in ventricular myocytes requires Na+-Ca2+ exchanger function
Abstract
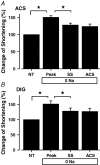
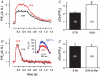
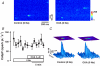
Glycoside-induced cardiac inotropy has traditionally been attributed to direct Na(+)-K(+)-ATPase inhibition, causing increased intracellular [Na(+)] and consequent Ca(2+) gain via the Na(+)-Ca(2+) exchanger (NCX). However, recent studies suggested alternative mechanisms of glycoside-induced inotropy: (1) direct activation of sarcoplasmic reticulum Ca(2+) release channels (ryanodine receptors; RyRs); (2) increased Ca(2+) selectivity of Na(+) channels (slip-mode conductance); and (3) other signal transduction pathways. None of these proposed mechanisms requires NCX or an altered [Na(+)] gradient. Here we tested the ability of ouabain (OUA, 3 microm), digoxin (DIG, 20 microm) or acetylstrophanthidin (ACS, 4 microm) to alter Ca(2+) transients in completely Na(+)-free conditions in intact ferret and cat ventricular myocytes. We also tested whether OUA directly activates RyRs in permeabilized cat myocytes (measuring Ca(2+) sparks by confocal microscopy). In intact ferret myocytes (stimulated at 0.2 Hz), DIG and ACS enhanced Ca(2+) transients and cell shortening during twitches, as expected. However, prior depletion of [Na(+)](i) (in Na(+)-free, Ca(2+)-free solution) and in Na(+)-free solution (replaced by Li(+)) the inotropic effects of DIG and ACS were completely prevented. In voltage-clamped cat myocytes, OUA increased Ca(2+) transients by 48 +/- 4% but OUA had no effect in Na(+)-depleted cells (replaced by N-methyl-d-glucamine). In permeabilized cat myocytes, OUA did not change Ca(2+) spark frequency, amplitude or spatial spread (although spark duration was slightly prolonged). We conclude that the acute inotropic effects of DIG, ACS and OUA (and the effects on RyRs) depend on the presence of Na(+) and a functional NCX in ferret and cat myocytes (rather than alternate Na(+)-independent mechanisms).
Figures

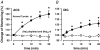
References
-
- Arnon A, Hamlyn JM, Blaustein MP. Ouabain augments Ca2+ transients in arterial smooth muscle without raising cytosolic Na+ Am J Physiol Heart Circ Physiol. 2000;279:H679–H691. - PubMed
-
- Bassani RA, Bassani JW, Bers DM. Relaxation in ferret ventricular myocytes: role of the sarcolemmal Ca2+ ATPase. Pflugers Arch. 1995;430:573–578. - PubMed
-
- Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. 2. Netherlands: Kluwer Academic Publishers; 2001.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

