Real-time PCR assays for hepatitis C virus (HCV) RNA quantitation are adequate for clinical management of patients with chronic HCV infection
- PMID: 16825372
- PMCID: PMC1489518
- DOI: 10.1128/JCM.00163-06
Real-time PCR assays for hepatitis C virus (HCV) RNA quantitation are adequate for clinical management of patients with chronic HCV infection
Abstract
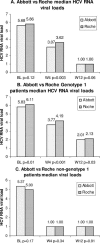
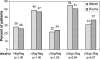
Because of the use of viral kinetics during polyethylene glycol (PEG)-interferon-ribavirin therapy and the development of specific new anti-hepatitis C virus (anti-HCV) drugs, assessment of the efficacy of anti-HCV drugs needs to be based not on end-point PCR assays but on real-time PCR. The aim of this study was to determine if the two available commercial real-time PCR assays, the Abbott RealTime HCV assay and the Roche Cobas TaqMan HCV assay, can become the standard for HCV RNA quantification. We investigated the prognostic relevance of HCV RNA viral loads at baseline, week 4, and week 12 to a rapid and early virological response to antiviral therapy by using the two assays. Of 59 naïve patients chronically infected by HCV (41 infected with genotype 1) who were treated with ribavirin plus PEG-interferon alfa-2b for 48 weeks, 24 patients (41%) showed a sustained virological response (SVR). With the two assays, viral loads were highly correlated, irrespective of genotype (R2=0.94 for all cases). No difference in diagnostic value was found between the Abbott and Roche assays at week 4, with respective negative predictive values (NPVs) of 84% and 78% and positive predictive values (PPVs) of 62% and 56% (not significant), and at week 12, the respective NPVs were 91% and 90% and PPVs were 44% and 46% (not significant). At week 12, 83% (20/24) and 96% (23/24) of patients with SVR tested negative for HCV RNA by the Abbott and Roche assays, respectively (the difference is not significant). In conclusion, the high sensitivities and large dynamic ranges of the Abbott and Roche assays show that a single real-time quantitative PCR assay is fully adequate for clinical and therapeutic management of HCV.
Figures



Similar articles
-
Assessment of early virological response to antiviral therapy by comparing four assays for HCV RNA quantitation using the international unit standard: implications for clinical management of patients with chronic hepatitis C virus infection.J Med Virol. 2006 Feb;78(2):208-15. doi: 10.1002/jmv.20529. J Med Virol. 2006. PMID: 16372298 Clinical Trial.
-
Abbott RealTime hepatitis C virus (HCV) and Roche Cobas AmpliPrep/Cobas TaqMan HCV assays for prediction of sustained virological response to pegylated interferon and ribavirin in chronic hepatitis C patients.J Clin Microbiol. 2009 Feb;47(2):385-9. doi: 10.1128/JCM.01753-08. Epub 2008 Dec 17. J Clin Microbiol. 2009. PMID: 19091819 Free PMC article.
-
Estimation of two real-time RT-PCR assays for quantitation of hepatitis C virus RNA during PEG-IFN plus ribavirin therapy by HCV genotypes and IL28B genotype.J Infect Chemother. 2013 Feb;19(1):63-9. doi: 10.1007/s10156-012-0452-1. Epub 2012 Jul 21. J Infect Chemother. 2013. PMID: 22821355 Clinical Trial.
-
Abbott RealTime PCR assay is useful for evaluating virological response to antiviral treatment for chronic hepatitis C.J Infect Chemother. 2011 Dec;17(6):737-43. doi: 10.1007/s10156-011-0249-7. Epub 2011 Apr 29. J Infect Chemother. 2011. PMID: 21528383
-
Treatment of chronic hepatitis C in Asia: when East meets West.J Gastroenterol Hepatol. 2009 Mar;24(3):336-45. doi: 10.1111/j.1440-1746.2009.05789.x. J Gastroenterol Hepatol. 2009. PMID: 19335784 Review.
Cited by
-
Influence of some methylated hepatocarcinogenesis-related genes on the response to antiviral therapy and development of fibrosis in chronic hepatitis C patients.Clin Mol Hepatol. 2020 Jan;26(1):60-69. doi: 10.3350/cmh.2019.0051. Epub 2019 Oct 22. Clin Mol Hepatol. 2020. PMID: 31630500 Free PMC article.
-
Amplification chemistries in clinical virology.J Clin Virol. 2019 Jun;115:18-31. doi: 10.1016/j.jcv.2019.03.015. Epub 2019 Mar 27. J Clin Virol. 2019. PMID: 30953805 Free PMC article. Review.
-
Occult HCV or delayed viral clearance from lymphocytes of Chronic HCV genotype 3 patients after interferon therapy.Genet Vaccines Ther. 2011 Sep 6;9(1):14. doi: 10.1186/1479-0556-9-14. Genet Vaccines Ther. 2011. PMID: 21892969 Free PMC article.
-
A novel diagnostic target in the hepatitis C virus genome.PLoS Med. 2009 Feb 10;6(2):e31. doi: 10.1371/journal.pmed.1000031. PLoS Med. 2009. PMID: 19209955 Free PMC article.
-
Evaluation of the Abbott investigational use only RealTime hepatitis C virus (HCV) assay and comparison to the Roche TaqMan HCV analyte-specific reagent assay.J Clin Microbiol. 2009 Sep;47(9):2872-8. doi: 10.1128/JCM.02329-08. Epub 2009 Jul 22. J Clin Microbiol. 2009. PMID: 19625475 Free PMC article.
References
-
- Afdhal, N., M. Rodriguez-Torres, and E. Lawitz. 2005. Enhanced antiviral efficacy for valopicitabine (NM283) plus peg-interferon in hepatitis C patients with HCV genotype-1 infection: results of a phase IIa multicenter trial. J. Hepatol. 42:39-40.
-
- Bergk, A., C. Sarrazin, G. Teuber, P. Buggisch, H. Klinker, V. Weich, B. Wiedenmann, and T. Berg. 2005. Detection of minimal residual hepatitis C viremia at treatment week 12 is associated with a high probability of relapse, abstr. 1188. Hepatology 42(Suppl. 1):663A-664A.
-
- Davis, G. L., J. B. Wong, J. G. McHutchison, M. P. Manns, J. Harvey, and J. Albrecht. 2003. Early virologic response to treatment with peginterferon alfa-2b plus ribavirin in patients with chronic hepatitis C. Hepatology 38:645-652. - PubMed
-
- European Association for the Study of the Liver. 1999. EASL International Consensus Conference on Hepatitis C. Paris, 26-28, February 1999, consensus statement. J. Hepatol. 30:956-961. - PubMed
-
- Ferenci, P., M. W. Fried, M. L. Shiffman, C. I. Smith, G. Marinos, F. L. Goncales, Jr., D. Haussinger, M. Diago, G. Carosi, D. Dhumeaux, A. Craxi, M. Chaneac, and K. R. Reddy. 2005. Predicting sustained virological responses in chronic hepatitis C patients treated with peginterferon alfa-2a (40 KD)/ribavirin. J. Hepatol. 43:425-433. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

