Use of in vitro assays to determine effects of human serum on biological characteristics of Acanthamoeba castellanii
- PMID: 16825391
- PMCID: PMC1489474
- DOI: 10.1128/JCM.00144-06
Use of in vitro assays to determine effects of human serum on biological characteristics of Acanthamoeba castellanii
Abstract
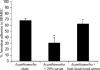
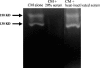
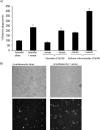
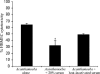
Normal human serum inhibits Acanthamoeba (encephalitis isolate) binding to and cytotoxicity of human brain microvascular endothelial cells, which constitute the blood-brain barrier. Zymographic assays revealed that serum inhibits extracellular protease activities of acanthamoebae. But it is most likely that inhibition of specific properties of acanthamoebae is a consequence of the initial amoebicidal-amoebistatic effects induced by serum. For example, serum exhibited amoebicidal effects; i.e., up to 50% of the exposed trophozoites were killed. The residual subpopulation, although viable, remained static over longer incubations. Interestingly, serum enhanced the phagocytic ability of acanthamoebae, as measured by bacterial uptake. Overall, our results demonstrate that human serum has inhibitory effects on Acanthamoeba growth and viability, protease secretions, and binding to and subsequent cytotoxicity for brain microvascular endothelial cells. Conversely, Acanthamoeba phagocytosis was stimulated by serum.
Figures


References
-
- Alsam, S., K. S. Kim, M. Stins, A. O. Rivas, J. Sissons, and N. A. Khan. 2003. Acanthamoeba interactions with human brain microvascular endothelial cells. Microb. Pathog. 35(6):235-241. - PubMed
-
- Alsam, S., J. J. Sissons, S. Jayasekera, and N. A. Khan. 2005. Extracellular proteases of Acanthamoeba castellanii (encephalitis isolate belonging to T1 genotype) contribute to increased permeability in an in vitro model of the human blood-brain barrier. J. Infect. 51(2):150-156. - PubMed
-
- Alsam, S., J. Sissons, R. Dudley, and N. A. Khan. 2005. Mechanisms associated with Acanthamoeba castellanii (T4) phagocytosis. Parasitol. Res. 96(6):402-409. - PubMed
-
- Ferrante, A. 1991. Immunity to Acanthamoeba. Rev. Infect. Dis. 13:S403-S409. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

