Cell-culture assays reveal the importance of retroviral vector design for insertional genotoxicity
- PMID: 16825499
- PMCID: PMC1895590
- DOI: 10.1182/blood-2005-08-024976
Cell-culture assays reveal the importance of retroviral vector design for insertional genotoxicity
Abstract
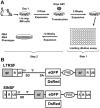
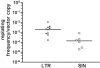
Retroviral vectors with long terminal repeats (LTRs), which contain strong enhancer/promoter sequences at both ends of their genome, are widely used for stable gene transfer into hematopoietic cells. However, recent clinical data and mouse models point to insertional activation of cellular proto-oncogenes as a dose-limiting side effect of retroviral gene delivery that potentially induces leukemia. Self-inactivating (SIN) retroviral vectors do not contain the terminal repetition of the enhancer/promoter, theoretically attenuating the interaction with neighboring cellular genes. With a new assay based on in vitro expansion of primary murine hematopoietic cells and selection in limiting dilution, we showed that SIN vectors using a strong internal retroviral enhancer/promoter may also transform cells by insertional mutagenesis. Most transformed clones, including those obtained after dose escalation of SIN vectors, showed insertions upstream of the third exon of Evi1 and in reverse orientation to its transcriptional orientation. Normalizing for the vector copy number, we found the transforming capacity of SIN vectors to be significantly reduced when compared with corresponding LTR vectors. Additional modifications of SIN vectors may further increase safety. Improved cell-culture assays will likely play an important role in the evaluation of insertional mutagenesis.
Figures

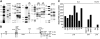

References
-
- Hacein-Bey-Abina S, Le Deist F, Carlier F, et al. Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy. N Engl J Med. 2002;346: 1185-1193. - PubMed
-
- Aiuti A, Slavin S, Aker M, et al. Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science. 2002; 296: 2410-2413. - PubMed
-
- Gaspar HB, Parsley KL, Howe S, et al. Gene therapy of X-linked severe combined immunodeficiency by use of a pseudotyped gammaretroviral vector. Lancet. 2004;364: 2181-2187. - PubMed
-
- Ott MG, Schmidt M, Schwarzwaelder K, et al. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1. Nat Med. 2006;12: 401-409. - PubMed
-
- Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. 2003;4: 346-358. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

