Increased sensitivity of the neuronal nicotinic receptor alpha 2 subunit causes familial epilepsy with nocturnal wandering and ictal fear
- PMID: 16826524
- PMCID: PMC1559502
- DOI: 10.1086/506459
Increased sensitivity of the neuronal nicotinic receptor alpha 2 subunit causes familial epilepsy with nocturnal wandering and ictal fear
Abstract
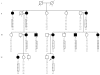
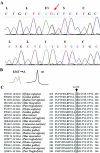
Sleep has traditionally been recognized as a precipitating factor for some forms of epilepsy, although differential diagnosis between some seizure types and parasomnias may be difficult. Autosomal dominant frontal lobe epilepsy is characterized by nocturnal seizures with hyperkinetic automatisms and poorly organized stereotyped movements and has been associated with mutations of the alpha 4 and beta 2 subunits of the neuronal nicotinic acetylcholine receptor. We performed a clinical and molecular genetic study of a large pedigree segregating sleep-related epilepsy in which seizures are associated with fear sensation, tongue movements, and nocturnal wandering, closely resembling nightmares and sleep walking. We identified a new genetic locus for familial sleep-related focal epilepsy on chromosome 8p12.3-8q12.3. By sequencing the positional candidate neuronal cholinergic receptor alpha 2 subunit gene (CHRNA2), we detected a heterozygous missense mutation, I279N, in the first transmembrane domain that is crucial for receptor function. Whole-cell recordings of transiently transfected HEK293 cells expressing either the mutant or the wild-type receptor showed that the new CHRNA2 mutation markedly increases the receptor sensitivity to acetylcholine, therefore indicating that the nicotinic alpha 2 subunit alteration is the underlying cause. CHRNA2 is the third neuronal cholinergic receptor gene to be associated with familial sleep-related epilepsies. Compared with the CHRNA4 and CHRNB2 mutations reported elsewhere, CHRNA2 mutations cause a more complex and finalized ictal behavior.
Figures


References
Web Resources
-
- Brain and Tissue Bank for Developmental Disorders, http://medschool.umaryland.edu/BTBank/
-
- GDB Human Genome Database, http://www.gdb.org/ (for allele frequencies)
-
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for CHRNA2 [accession number NM_000742] and CHRNB4 [accession number NM_000750])
-
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for ADNFLE) - PubMed
-
- UCSC Genome Browser, http://genome.ucsc.edu/ (for selecting critical region candidate genes)
References
-
- Steinlein O, Sander T, Stoodt J, Kretz R, Janz D, Propping P (1997) Possible association of a silent polymorphism in the neuronal nicotinic acetylcholine receptor subunit α4 with common idiopathic generalized epilepsies. Am J Med Genet 74:445–44910.1002/(SICI)1096-8628(19970725)74:4<445::AID-AJMG18>3.0.CO;2-I - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

