Design of immunogens that present the crown of the HIV-1 V3 loop in a conformation competent to generate 447-52D-like antibodies
- PMID: 16827663
- PMCID: PMC1615908
- DOI: 10.1042/BJ20060588
Design of immunogens that present the crown of the HIV-1 V3 loop in a conformation competent to generate 447-52D-like antibodies
Abstract
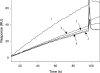
gp120 is a subunit of the envelope glycoprotein of HIV-1. The third variable loop region of gp120 (V3 loop) contains multiple immunodominant epitopes and is also functionally important for deciding cell-tropism of the virus. 447-52D is a monoclonal antibody that recognizes the conserved tip of the V3 loop in a beta-turn conformation. This antibody has previously been shown to neutralize diverse strains of the virus. In an attempt to generate an immunogen competent to generate 447-52D-like antibodies, the known epitope of 447-52D was inserted at three different surface loop locations in the small, stable protein Escherichia coli Trx (thioredoxin). At one of the three locations (between residues 74 and 75), the insertion was tolerated, the resulting protein was stable and soluble, and bound 447-52D with an affinity similar to that of intact gp120. Upon immunization, the V3 peptide-inserted Trx scaffold was able to generate anti-V3 antibodies that could compete out 447-52D binding to gp120. Epitope mapping studies demonstrated that these anti-V3 antibodies recognized the same epitope as 447-52D. Although the 447-52D-type antibodies were estimated to be present at concentrations of 50-400 microg/ml of serum, these were not able to effect neutralization of strains like JRFL and BAL but could neutralize the sensitive MN strain. The data suggest that because of the low accessibility of the V3 loop on primary isolates such as JRFL, it will be difficult to elicit a V3-specific, 447-52D-like antibody response to effectively neutralize such isolates.
Figures
Similar articles
-
The effect of low-profile serine substitutions in the V3 loop of HIV-1 gp120 IIIB/LAI on the immunogenicity of the envelope protein.Virology. 1998 Nov 10;251(1):59-70. doi: 10.1006/viro.1998.9392. Virology. 1998. PMID: 9813203
-
Induced fit in HIV-neutralizing antibody complexes: evidence for alternative conformations of the gp120 V3 loop and the molecular basis for broad neutralization.Biochemistry. 2005 May 17;44(19):7250-8. doi: 10.1021/bi047387t. Biochemistry. 2005. PMID: 15882063
-
Binding of antibodies to virion-associated gp120 molecules of primary-like human immunodeficiency virus type 1 (HIV-1) isolates: effect on HIV-1 infection of macrophages and peripheral blood mononuclear cells.Virology. 1997 Mar 17;229(2):360-9. doi: 10.1006/viro.1997.8443. Virology. 1997. PMID: 9126249
-
Structural studies of human HIV-1 V3 antibodies.Hum Antibodies. 2005;14(3-4):73-80. Hum Antibodies. 2005. PMID: 16720977 Review.
-
[Role of the HIV-1 gp120 V1/V2 domains in the induction of neutralizing antibodies].Enferm Infecc Microbiol Clin. 2009 Nov;27(9):523-30. doi: 10.1016/j.eimc.2008.02.010. Epub 2009 May 1. Enferm Infecc Microbiol Clin. 2009. PMID: 19409660 Review. Spanish.
Cited by
-
An oligosaccharide-based HIV-1 2G12 mimotope vaccine induces carbohydrate-specific antibodies that fail to neutralize HIV-1 virions.Proc Natl Acad Sci U S A. 2008 Oct 14;105(41):15684-9. doi: 10.1073/pnas.0807837105. Epub 2008 Oct 6. Proc Natl Acad Sci U S A. 2008. PMID: 18838688 Free PMC article.
-
T = 4 Icosahedral HIV-1 Capsid As an Immunogenic Vector for HIV-1 V3 Loop Epitope Display.Viruses. 2018 Nov 26;10(12):667. doi: 10.3390/v10120667. Viruses. 2018. PMID: 30486318 Free PMC article.
-
Protein engineering strategies for the development of viral vaccines and immunotherapeutics.FEBS Lett. 2014 Jan 21;588(2):298-307. doi: 10.1016/j.febslet.2013.10.014. Epub 2013 Oct 21. FEBS Lett. 2014. PMID: 24157357 Free PMC article. Review.
-
Transplanting supersites of HIV-1 vulnerability.PLoS One. 2014 Jul 3;9(7):e99881. doi: 10.1371/journal.pone.0099881. eCollection 2014. PLoS One. 2014. PMID: 24992528 Free PMC article.
-
Expression, glycoform characterization, and antibody-binding of HIV-1 V3 glycopeptide domain fused with human IgG1-Fc.Bioconjug Chem. 2010 May 19;21(5):875-83. doi: 10.1021/bc9004238. Bioconjug Chem. 2010. PMID: 20369886 Free PMC article.
References
-
- Binley J. M., Wrin T., Korber B., Zwick M. B., Wang M., Chappey C., Stiegler G., Kunert R., Zolla-Pazner S., Katinger H., et al. Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J. Virol. 2004;78:13232–13252. - PMC - PubMed
-
- Vogel T., Kurth R., Norley S. The majority of neutralizing Abs in HIV-1-infected patients recognize linear V3 loop sequences. Studies using HIV-1MN multiple antigenic peptides. J. Immunol. 1994;153:1895–1904. - PubMed
-
- Zwart G., Back N. K., Ramautarsing C., Valk M., van der Hoek L., Goudsmit J. Frequent and early HIV-1MN neutralizing capacity in sera from Dutch HIV-1 seroconverters is related to antibody reactivity to peptides from the gp120 V3 domain. AIDS Res. Hum. Retroviruses. 1994;10:245–251. - PubMed
-
- Javaherian K., Langlois A. J., McDanal C., Ross K. L., Eckler L. I., Jellis C. L., Profy A. T., Rusche J. R., Bolognesi D. P., Putney S. D., et al. Principal neutralizing domain of the human immunodeficiency virus type 1 envelope protein. Proc. Natl. Acad. Sci. U.S.A. 1989;86:6768–6772. - PMC - PubMed
-
- Palker T. J., Matthews T. J., Langlois A., Tanner M. E., Martin M. E., Scearce R. M., Kim J. E., Berzofsky J. A., Bolognesi D. P., Haynes B. F. Polyvalent human immunodeficiency virus synthetic immunogen comprised of envelope gp120 T helper cell sites and B cell neutralization epitopes. J. Immunol. 1989;142:3612–3619. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

