Synthetic small interfering RNA targeting heat shock protein 105 induces apoptosis of various cancer cells both in vitro and in vivo
- PMID: 16827803
- PMCID: PMC11159471
- DOI: 10.1111/j.1349-7006.2006.00217.x
Synthetic small interfering RNA targeting heat shock protein 105 induces apoptosis of various cancer cells both in vitro and in vivo
Abstract
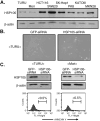
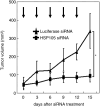
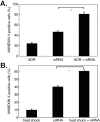
We previously reported that heat shock protein 105 (HSP105), identified by serological analysis of a recombinant cDNA expression library (SEREX) using serum from a pancreatic cancer patient, was overexpressed in various human tumors and in the testis of adult men by immunohistochemical analysis. In the present study, to elucidate the biological function of the HSP105 protein in cancer cells, we first established NIH3T3 cells overexpressing murine HSP105 (NIH3T3-HSP105). The NIH3T3-HSP105 cells acquired resistance to apoptosis induced by heat shock or doxorubicin. The small interfering RNA (siRNA)-mediated suppression of HSP105 protein expression induced apoptosis in human cancer cells but not in fibroblasts. By a combination of siRNA introduction and doxorubicin or heat shock treatment, apoptosis was induced synergistically in a human colon cancer cell line, HCT116. In vivo, siRNA inoculation into the human gastric cancer cell line KATO-3 established in the flank of an NOD SCID mouse suppressed the tumor growth. This siRNA-induced apoptosis was mediated through caspases, but not the p53 tumor suppressor protein, even though the HSP105 protein was bound to wild-type p53 protein in HCT116 cells. These findings suggest that the constitutive overexpression of HSP105 in cancer cells is involved in malignant transformation by protecting tumor cells from apoptosis. HSP105 may thus be a novel target molecule for cancer therapy and a treatment regimen using synthetic siRNA to suppress the expression of HSP105 protein may provide a new strategy for cancer therapy.
Figures



Similar articles
-
Heat shock protein 105 is overexpressed in a variety of human tumors.Oncol Rep. 2003 Nov-Dec;10(6):1777-82. Oncol Rep. 2003. PMID: 14534695
-
Cloning and expression of murine high molecular mass heat shock proteins, HSP105.J Biol Chem. 1995 Dec 15;270(50):29718-23. doi: 10.1074/jbc.270.50.29718. J Biol Chem. 1995. PMID: 8530361
-
DNA vaccination of HSP105 leads to tumor rejection of colorectal cancer and melanoma in mice through activation of both CD4 T cells and CD8 T cells.Cancer Sci. 2005 Oct;96(10):695-705. doi: 10.1111/j.1349-7006.2005.00093.x. Cancer Sci. 2005. PMID: 16232202 Free PMC article.
-
Characterization of stress sensitivity and chaperone activity of Hsp105 in mammalian cells.Biochem Biophys Res Commun. 2011 May 27;409(1):90-5. doi: 10.1016/j.bbrc.2011.04.114. Epub 2011 May 1. Biochem Biophys Res Commun. 2011. PMID: 21557931
-
Exploration of small-molecule inhibitors targeting Hsp110 as novel therapeutics.Drug Discov Today. 2025 Jan;30(1):104287. doi: 10.1016/j.drudis.2024.104287. Epub 2025 Jan 3. Drug Discov Today. 2025. PMID: 39756648 Review.
Cited by
-
Identification of HLA-A2 or HLA-A24-restricted CTL epitopes for potential HSP105-targeted immunotherapy in colorectal cancer.Oncol Rep. 2014 Mar;31(3):1051-8. doi: 10.3892/or.2013.2941. Epub 2013 Dec 20. Oncol Rep. 2014. PMID: 24366042 Free PMC article.
-
HSP105 expression in cutaneous malignant melanoma: Correlation with clinicopathological characteristics.PLoS One. 2021 Oct 7;16(10):e0258053. doi: 10.1371/journal.pone.0258053. eCollection 2021. PLoS One. 2021. PMID: 34618840 Free PMC article.
-
The Interplay between Heat Shock Proteins and Cancer Pathogenesis: A Novel Strategy for Cancer Therapeutics.Cancers (Basel). 2024 Feb 1;16(3):638. doi: 10.3390/cancers16030638. Cancers (Basel). 2024. PMID: 38339390 Free PMC article. Review.
-
HSP105 suppresses the progression of cutaneous squamous cell carcinoma by activating the P53 signaling pathway.Am J Cancer Res. 2023 Jul 15;13(7):3013-3026. eCollection 2023. Am J Cancer Res. 2023. PMID: 37559974 Free PMC article.
-
Single-Cell Sequencing to Identify Six Heat Shock Protein (HSP) Genes-Mediated Progression Subtypes of Clear Cell Renal Cell Carcinoma.Int J Gen Med. 2021 Jul 23;14:3761-3773. doi: 10.2147/IJGM.S318271. eCollection 2021. Int J Gen Med. 2021. PMID: 34326662 Free PMC article.
References
-
- Lee‐Yoon D, Easton D, Murawski M, Burd R, Subjeck JR. Identification of a major subfamily of large hsp70‐like proteins through the cloning of the mammalian 110‐kDa heat shock protein. J Biol Chem 1995; 270: 15 725–33. - PubMed
-
- Yasuda K, Nakai A, Hatayama T, Nagata K. Cloning and expression of murine high molecular mass heat shock proteins, HSP105. J Biol Chem 1995; 270: 29 718–23. - PubMed
-
- Wakatsuki T, Hatayama T. Characteristic expression of 105‐kDa heat shock protein (HSP105) in various tissues of non‐stressed and heat‐stressed rats. Biol Pharm Bull 1998; 21: 905–10. - PubMed
-
- Satoh J, Yukitake M, Kuroda Y. Constitutive and heat‐inducible expression of HSP105 in neurons and glial cells in culture. Neuroreport 1998; 9: 2977–83. - PubMed
-
- Ishihara K, Yasuda K, Hatayama T. Molecular cloning, expression and localization of human 105 kDa heat shock protein, hsp105. Biochim Biophys Acta 1999; 1444: 138–42. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

