The fate of effector CD8 T cells in vivo is controlled by the duration of antigen stimulation
- PMID: 16827897
- PMCID: PMC1782300
- DOI: 10.1111/j.1365-2567.2006.02381.x
The fate of effector CD8 T cells in vivo is controlled by the duration of antigen stimulation
Abstract
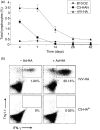
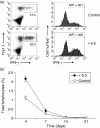
What controls the fate of the T-cell response remains incompletely defined. Gain of effector function facilitated by costimulation has been thought to be a crucial factor in determining the outcome of the T-cell response, i.e. long-term memory in the presence of costimulation versus tolerance induction in the absence of costimulation. In this study, we show that while costimulation or cognate CD4 helps to promote the acquisition of effector function during the initial phase of the CD8 T-cell response, the fate of effector CD8 T cells is controlled by the duration of subsequent antigenic stimulation. Effector CD8 T cells differentiate into memory cells only after clearance of antigen, whereas in the presence of persistent antigen, effector CD8 T cells are tolerized. Furthermore, protective immunity against tumour cannot develop in the persisting antigen environment. These results suggest that removal of persisting antigen by other means might be a prerequisite for effective immunotherapy in cancer.
Figures






References
-
- Lafferty KJ, Cunningham AJ. A new analysis of allogeneic interactions. Aust J Exp Biol Med Sci. 1975;53:27–42. - PubMed
-
- Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–8. - PubMed
-
- Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

