A dual altered peptide ligand down-regulates myasthenogenic T cell responses and reverses experimental autoimmune myasthenia gravis via up-regulation of Fas-FasL-mediated apoptosis
- PMID: 16827902
- PMCID: PMC1782294
- DOI: 10.1111/j.1365-2567.2006.02398.x
A dual altered peptide ligand down-regulates myasthenogenic T cell responses and reverses experimental autoimmune myasthenia gravis via up-regulation of Fas-FasL-mediated apoptosis
Abstract
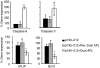
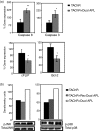
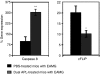
Myasthenia gravis (MG) and experimental autoimmune MG (EAMG) are T cell-dependent, antibody-mediated autoimmune diseases. A dual altered peptide ligand (APL) that is composed of the tandemly arranged two single amino acid analogues of two myasthenogenic peptides, p195-212 and p259-271, was demonstrated to down-regulate in vitro and in vivo MG-associated autoreactive responses. The aims of this study were to investigate the possible role of Fas-FasL-mediated apoptosis in the down-regulatory mechanism of the dual APL. We demonstrate here the effect of the dual APL on expression of key molecules involved in the Fas-FasL pathway, in a p195-212-specific T cell line, in mice immunized with Torpedo acetylcholine receptor and in mice afflicted with EAMG (induced with the latter). In vitro and in vivo results show that the dual APL up-regulated expression of Fas and FasL on the CD4 cells. Expression of the pro-apoptotic molecules, caspase 8 and caspase 3, was significantly up-regulated, while anti-apoptotic cFLIP and Bcl-2 were down-regulated upon treatment with the dual APL. The dual APL also increased phosphorylation of the mitogen-activated protein kinases, c-Jun-NH2-terminal kinase and p-38, known to play a role in the regulation of FasL expression. Further, in the T cell line incubated with the dual APL as well as in mice of the SJL inbred strain immunized with the myasthenogenic peptide and treated concomitantly with the dual APL, the percentage of apoptotic cells increased. Results strongly indicate that up-regulation of apoptosis via the Fas-FasL pathway is one of the mechanisms by which the dual APL reverses EAMG manifestations in C57BL/6 mice.
Figures

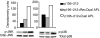




Similar articles
-
A dual altered peptide ligand inhibits myasthenia gravis associated responses by inducing phosphorylated extracellular-regulated kinase 1,2 that upregulates CD4+CD25+Foxp3+ cells.Scand J Immunol. 2007 Jun;65(6):567-76. doi: 10.1111/j.1365-3083.2007.01940.x. Scand J Immunol. 2007. PMID: 17523950
-
Down-regulation of T cell responses to AChR and reversal of EAMG manifestations in mice by a dual altered peptide ligand via induction of CD4+ CD25+ regulatory cells.J Neuroimmunol. 2006 Aug;177(1-2):63-75. doi: 10.1016/j.jneuroim.2006.04.018. Epub 2006 Jun 6. J Neuroimmunol. 2006. PMID: 16757035
-
A dual altered peptide ligand down-regulates myasthenogenic T cell responses by up-regulating CD25- and CTLA-4-expressing CD4+ T cells.Proc Natl Acad Sci U S A. 2003 May 27;100(11):6676-81. doi: 10.1073/pnas.1131898100. Epub 2003 May 12. Proc Natl Acad Sci U S A. 2003. PMID: 12743364 Free PMC article.
-
On the role and significance of Fas (Apo-1/CD95) ligand (FasL) expression in immune privileged tissues and cancer cells using multiple myeloma as a model.Leuk Lymphoma. 1998 Nov;31(5-6):477-90. doi: 10.3109/10428199809057607. Leuk Lymphoma. 1998. PMID: 9922038 Review.
-
Modification of human T-cell responses by altered peptide ligands: a new approach to antigen-specific modification.Intern Med. 1998 Oct;37(10):804-17. doi: 10.2169/internalmedicine.37.804. Intern Med. 1998. PMID: 9840700 Review.
Cited by
-
MiR-320a is downregulated in patients with myasthenia gravis and modulates inflammatory cytokines production by targeting mitogen-activated protein kinase 1.J Clin Immunol. 2013 Apr;33(3):567-76. doi: 10.1007/s10875-012-9834-5. Epub 2012 Nov 30. J Clin Immunol. 2013. PMID: 23196978
-
Peptide Dose and/or Structure in Vaccines as a Determinant of T Cell Responses.Vaccines (Basel). 2014 Jul 2;2(3):537-48. doi: 10.3390/vaccines2030537. Vaccines (Basel). 2014. PMID: 26344744 Free PMC article. Review.
-
A broadly applicable approach to T cell epitope identification: application to improving tumor associated epitopes and identifying epitopes in complex pathogens.J Immunol Methods. 2011 Oct 28;373(1-2):111-26. doi: 10.1016/j.jim.2011.08.007. Epub 2011 Aug 18. J Immunol Methods. 2011. PMID: 21872603 Free PMC article.
-
FOSL1-mediated LINC01566 negatively regulates CD4+ T-cell activation in myasthenia gravis.J Neuroinflammation. 2024 Aug 7;21(1):197. doi: 10.1186/s12974-024-03194-5. J Neuroinflammation. 2024. PMID: 39113081 Free PMC article.
-
Pathological Findings in Myasthenia Gravis Patients with Thymic Hyperplasia and Thymoma.Pathol Oncol Res. 2018 Jan;24(1):67-74. doi: 10.1007/s12253-017-0213-7. Epub 2017 Mar 15. Pathol Oncol Res. 2018. PMID: 28299711
References
-
- Lindstrom J, Shelton D, Fuji Y. Myasthenia gravis. Adv Immunol. 1988;42:233–84. - PubMed
-
- Drachman DB. Medical progress: myasthenia gravis. N Engl J Med. 1994;330:1797–810. - PubMed
-
- Brocke S, Brautbar C, Steinman L, Abramsky O, Rothbard J, Neumann D, Fuchs S, Mozes E. In vitro proliferative responses and antibody titers specific to human acetylcholine receptor synthetic peptides in patients with myasthenia gravis and relation to HLA class II genes. J Clin Invest. 1988;82:1894–900. - PMC - PubMed
-
- Karni A, Zisman E, Katz-Levy Y, et al. Reactivity of T cells from seronegative patients with myasthenia gravis to T cell epitopes of the human acetylcholine receptor. Neurology. 1997;48:1638–42. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

