Synthesis and evaluation of 3-aminopropionyl substituted fentanyl analogues for opioid activity
- PMID: 16828552
- PMCID: PMC1783977
- DOI: 10.1016/j.bmcl.2006.06.040
Synthesis and evaluation of 3-aminopropionyl substituted fentanyl analogues for opioid activity
Abstract
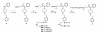
An enkephalin analogue coupled to 'aminofentanyl' has been synthesized and tested for biological activities at the mu and delta opioid receptors. Aminofentanyl which represents a structural derivative of fentanyl has been synthesized by acylation of 1-(2-phenethyl)-4-(N-anilino)piperidine with phthaloyl protected beta-alaninyl chloride in the presence of DIPEA, followed by deprotection with hydrazine hydrate. Aminofentanyl has also been successfully acylated with ethyl isocyanate, various acid anhydrides, to further investigate structure-activity relationships of these new fentanyl derivatives. Among the new derivatives compound 7 which carries a Tyr-D-Ala-Gly-Phe opioid message sequence showed good opioid affinity (1 nM at both delta and mu opioid receptors) and bioactivity (34.9 nM in MVD and 42 nM in GPI/LMMP bioassays).
Figures
Similar articles
-
Probes for narcotic receptor-mediated phenomena. 25. Synthesis and evaluation of N-alkyl-substituted (alpha-piperazinylbenzyl)benzamides as novel, highly selective delta opioid receptor agonists.J Med Chem. 1997 Aug 29;40(18):2936-47. doi: 10.1021/jm970106d. J Med Chem. 1997. PMID: 9288176
-
Selective opioid dipeptides.Biochem Biophys Res Commun. 1994 Feb 15;198(3):933-9. doi: 10.1006/bbrc.1994.1133. Biochem Biophys Res Commun. 1994. PMID: 8117299
-
Evolution of the Dmt-Tic pharmacophore: N-terminal methylated derivatives with extraordinary delta opioid antagonist activity.J Med Chem. 1997 Sep 12;40(19):3100-8. doi: 10.1021/jm9607663. J Med Chem. 1997. PMID: 9301674
-
Fentanyl-related compounds and derivatives: current status and future prospects for pharmaceutical applications.Future Med Chem. 2014 Mar;6(4):385-412. doi: 10.4155/fmc.13.215. Future Med Chem. 2014. PMID: 24635521 Free PMC article. Review.
-
Fentanyl Structure as a Scaffold for Opioid/Non-Opioid Multitarget Analgesics.Int J Mol Sci. 2022 Mar 2;23(5):2766. doi: 10.3390/ijms23052766. Int J Mol Sci. 2022. PMID: 35269909 Free PMC article. Review.
Cited by
-
Development of novel enkephalin analogues that have enhanced opioid activities at both mu and delta opioid receptors.J Med Chem. 2007 Nov 1;50(22):5528-32. doi: 10.1021/jm061465o. Epub 2007 Oct 10. J Med Chem. 2007. PMID: 17927164 Free PMC article.
-
Understanding the structural requirements of 4-anilidopiperidine analogues for biological activities at mu and delta opioid receptors.Bioorg Med Chem Lett. 2007 Apr 15;17(8):2161-5. doi: 10.1016/j.bmcl.2007.01.114. Epub 2007 Feb 8. Bioorg Med Chem Lett. 2007. PMID: 17329100 Free PMC article.
-
Design and synthesis of novel bivalent ligands (MOR and DOR) by conjugation of enkephalin analogues with 4-anilidopiperidine derivatives.Bioorg Med Chem Lett. 2015 Oct 15;25(20):4683-8. doi: 10.1016/j.bmcl.2015.07.064. Epub 2015 Jul 29. Bioorg Med Chem Lett. 2015. PMID: 26323872 Free PMC article.
-
Enkephalin analogues with N-phenyl-N-(piperidin-2-ylmethyl)propionamide derivatives: Synthesis and biological evaluations.Bioorg Med Chem Lett. 2016 Jan 1;26(1):222-7. doi: 10.1016/j.bmcl.2015.10.081. Epub 2015 Nov 3. Bioorg Med Chem Lett. 2016. PMID: 26611918 Free PMC article.
-
Novel multiple opioid ligands based on 4-aminobenzazepinone (Aba), azepinoindole (Aia) and tetrahydroisoquinoline (Tic) scaffolds.Bioorg Med Chem Lett. 2010 Mar 1;20(5):1610-3. doi: 10.1016/j.bmcl.2010.01.055. Epub 2010 Jan 20. Bioorg Med Chem Lett. 2010. PMID: 20137938 Free PMC article.
References
-
- Janssen PAJ, Gardocki JF. U.S. Patent 3141823 1964.
- Janssen PAJ. Fr. Patent M2430 1964.
-
- Riley TN, Hale DB, Wilson MC. J. Pharm. Sci. 1973;62:983. - PubMed
-
- Janssen PAJ, Van Daele GHP. Deutch. Patent 2610228 1976.
-
- Leysen JE, Laduron PM, Niemegeers CJE. In: Characteristics and Function of Opioids. Van Ree J, Terenius L, editors. Elsevier; North Holland: 1978. pp. 479–482.
-
- Van Bever WFM, Niemegeers CJE, Schellekens KHL, Janssen PAJ. Arzneim.-Forsch. 1976;26:1548. - PubMed
- Janssen PAJ, Van Daele GHP. U.S. Patent 4179569 1979.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Chemical Information
Research Materials

