Determining selectivity of phosphoinositide-binding domains
- PMID: 16829131
- PMCID: PMC3786563
- DOI: 10.1016/j.ymeth.2006.05.006
Determining selectivity of phosphoinositide-binding domains
Abstract
The burgeoning of phosphoinositide-binding domains and proteins in cellular signaling and trafficking has drawn laboratories from a wide variety of fields into the study of lipid interactions with peripheral membrane proteins. Many different approaches have been developed to assess phosphoinositide binding, some of which are more problematic than others, and some of which can be quantitated more readily than others. With a focus on the methods used in our laboratory, we describe here the considerations that need to be taken into account when establishing-and quantitating-the specific binding of a protein or domain to phosphoinositides in membranes. We also discuss briefly a few examples in which no clear consensus has yet been reached as to the specificity of a given domain or protein because of discrepancies between different commonly used approaches.
Figures
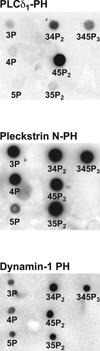


References
-
- Lemmon MA. Traffic. 2003;4:201–213. - PubMed
-
- DiNitto JP, Cronin TC, Lambright DG. Sci. STKE 2003. 2003:re16. - PubMed
-
- Yu JW, Lemmon MA. Curr. Opin. Chem. Biol. 2003;7:103–109. - PubMed
-
- Cho W, Stahelin RV. Annu. Rev. Biophys. Biomol. Struct. 2005;34:119–151. - PubMed
-
- Downes CP, Gray A, Lucocq JM. Trends Cell Biol. 2005;15:259–268. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

