Conformational changes in actin filaments induced by formin binding to the barbed end
- PMID: 16829561
- PMCID: PMC1562385
- DOI: 10.1529/biophysj.106.087775
Conformational changes in actin filaments induced by formin binding to the barbed end
Abstract
Formins bind actin filaments and play an essential role in the regulation of the actin cytoskeleton. In this work we describe details of the formin-induced conformational changes in actin filaments by fluorescence-lifetime and anisotropy-decay experiments. The results show that the binding of the formin homology 2 domain of a mammalian formin (mouse mDia1) to actin filaments resulted in a less rigid protein structure in the microenvironment of the Cys374 of actin, weakening of the interactions between neighboring actin protomers, and greater overall flexibility of the actin filaments. The formin effect is smaller at greater ionic strength. The results show that formin binding to the barbed end of actin filaments is responsible for the increase of flexibility of actin filaments. One formin dimer can affect the dynamic properties of an entire filament. Analyses of the results obtained at various formin/actin concentration ratios indicate that at least 160 actin protomers are affected by the binding of a single formin dimer to the barbed end of a filament.
Figures
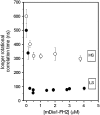
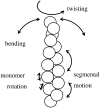
Similar articles
-
Formins regulate actin filament flexibility through long range allosteric interactions.J Biol Chem. 2006 Apr 21;281(16):10727-36. doi: 10.1074/jbc.M510252200. Epub 2006 Feb 20. J Biol Chem. 2006. PMID: 16490788 Free PMC article.
-
Myosin and tropomyosin stabilize the conformation of formin-nucleated actin filaments.J Biol Chem. 2012 Sep 14;287(38):31894-904. doi: 10.1074/jbc.M112.341230. Epub 2012 Jun 29. J Biol Chem. 2012. PMID: 22753415 Free PMC article.
-
The mouse formin, FRLalpha, slows actin filament barbed end elongation, competes with capping protein, accelerates polymerization from monomers, and severs filaments.J Biol Chem. 2004 May 7;279(19):20076-87. doi: 10.1074/jbc.M312718200. Epub 2004 Feb 29. J Biol Chem. 2004. PMID: 14990563
-
mDia1 and formins: screw cap of the actin filament.Biophysics (Nagoya-shi). 2012 May 31;8:95-102. doi: 10.2142/biophysics.8.95. eCollection 2012. Biophysics (Nagoya-shi). 2012. PMID: 27493525 Free PMC article. Review.
-
Regulators of actin filament barbed ends at a glance.J Cell Sci. 2016 Mar 15;129(6):1085-91. doi: 10.1242/jcs.179994. Epub 2016 Mar 3. J Cell Sci. 2016. PMID: 26940918 Review.
Cited by
-
Cooperative bundling by fascin generates actin structures with architectures that depend on filament length.Front Cell Dev Biol. 2022 Sep 2;10:974047. doi: 10.3389/fcell.2022.974047. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36120572 Free PMC article.
-
Interaction of formin FH2 with skeletal muscle actin. EPR and DSC studies.Eur Biophys J. 2013 Oct;42(10):757-65. doi: 10.1007/s00249-013-0922-0. Epub 2013 Aug 15. Eur Biophys J. 2013. PMID: 23949957 Free PMC article.
-
Helical rotation of the diaphanous-related formin mDia1 generates actin filaments resistant to cofilin.Proc Natl Acad Sci U S A. 2018 May 29;115(22):E5000-E5007. doi: 10.1073/pnas.1803415115. Epub 2018 May 14. Proc Natl Acad Sci U S A. 2018. PMID: 29760064 Free PMC article.
-
The Mechanisms of Thin Filament Assembly and Length Regulation in Muscles.Int J Mol Sci. 2022 May 10;23(10):5306. doi: 10.3390/ijms23105306. Int J Mol Sci. 2022. PMID: 35628117 Free PMC article. Review.
-
Role of tropomyosin in formin-mediated contractile ring assembly in fission yeast.Mol Biol Cell. 2009 Apr;20(8):2160-73. doi: 10.1091/mbc.e08-12-1201. Epub 2009 Feb 25. Mol Biol Cell. 2009. PMID: 19244341 Free PMC article.
References
-
- Wallar, B. J., and A. S. Alberts. 2003. The formins: active scaffolds that remodel the cytoskeleton. Trends Cell Biol. 13:435–446. - PubMed
-
- Wasserman, S. 1998. FH proteins as cytoskeletal organizers. Trends Cell Biol. 8:111–115. - PubMed
-
- Evangelista, M., D. Pruyne, D. C. Amberg, C. Boone, and A. Bretscher. 2002. Formins direct Arp2/3-independent actin filament assembly to polarize cell growth in yeast. Nat. Cell Biol. 4:32–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

