Hydrogen-bond switching through a radical pair mechanism in a flavin-binding photoreceptor
- PMID: 16829579
- PMCID: PMC1544145
- DOI: 10.1073/pnas.0600720103
Hydrogen-bond switching through a radical pair mechanism in a flavin-binding photoreceptor
Abstract
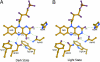
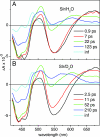
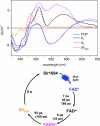
BLUF (blue light sensing using FAD) domains constitute a recently discovered class of photoreceptor proteins found in bacteria and eukaryotic algae, where they control a range of physiological responses including photosynthesis gene expression, photophobia, and negative phototaxis. Other than in well known photoreceptors such as the rhodopsins and phytochromes, BLUF domains are sensitive to light through an oxidized flavin rather than an isomerizable cofactor. To understand the physicochemical basis of BLUF domain photoactivation, we have applied femtosecond transient absorption spectroscopy to the Slr1694 BLUF domain of Synechocystis PCC6803. We show that photoactivation of BLUF domains proceeds by means of a radical-pair mechanism, driven by electron and proton transfer from the protein to the flavin, resulting in the transient formation of anionic and neutral flavin radical species that finally result in the long-lived signaling state on a 100-ps timescale. A pronounced deuteration effect is observed on the lifetimes of the intermediate radical species, indicating that proton movements underlie their molecular transformations. We propose a photoactivation mechanism that involves a successive rupture of hydrogen bonds between a conserved tyrosine and glutamine by light-induced electron transfer from tyrosine to flavin and between the glutamine and flavin by subsequent protonation at flavin N5. These events allow a reorientation of the conserved glutamine, resulting in a switching of the hydrogen-bond network connecting the chromophore to the protein, followed by radical-pair recombination, which locks the glutamine in place. It is suggested that the redox potential of flavin generally defines the light sensitivity of flavin-binding photoreceptors.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures


References
-
- van der Horst M. A., Hellingwerf K. J. Acc. Chem. Res. 2004;37:13–20. - PubMed
-
- Christie J. M., Briggs W. R. In: Handbook of Photosensory Receptors. Briggs W. R., Spudich J. L., editors. Weinheim, Germany: Wiley; 2005. pp. 277–304.
-
- Sancar A. Chem. Rev. 2003;103:2203–2237. - PubMed
-
- Batschauer A. In: Handbook of Photosensory Receptors. Briggs W. R., Spudich J. L., editors. Weinheim, Germany: Wiley; 2005. pp. 211–236.
-
- Masuda S., Bauer C. E. Cell. 2002;110:613–623. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

