Single molecules of the bacterial actin MreB undergo directed treadmilling motion in Caulobacter crescentus
- PMID: 16829583
- PMCID: PMC1544151
- DOI: 10.1073/pnas.0604503103
Single molecules of the bacterial actin MreB undergo directed treadmilling motion in Caulobacter crescentus
Abstract
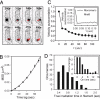
The actin cytoskeleton represents a key regulator of multiple essential cellular functions in both eukaryotes and prokaryotes. In eukaryotes, these functions depend on the orchestrated dynamics of actin filament assembly and disassembly. However, the dynamics of the bacterial actin homolog MreB have yet to be examined in vivo. In this study, we observed the motion of single fluorescent MreB-yellow fluorescent protein fusions in living Caulobacter cells in a background of unlabeled MreB. With time-lapse imaging, polymerized MreB [filamentous MreB (fMreB)] and unpolymerized MreB [globular MreB (gMreB)] monomers could be distinguished: gMreB showed fast motion that was characteristic of Brownian diffusion, whereas the labeled molecules in fMreB displayed slow, directed motion. This directional movement of labeled MreB in the growing polymer provides an indication that, like actin, MreB monomers treadmill through MreB filaments by preferential polymerization at one filament end and depolymerization at the other filament end. From these data, we extract several characteristics of single MreB filaments, including that they are, on average, much shorter than the cell length and that the direction of their polarized assembly seems to be independent of the overall cellular polarity. Thus, MreB, like actin, exhibits treadmilling behavior in vivo, and the long MreB structures that have been visualized in multiple bacterial species seem to represent bundles of short filaments that lack a uniform global polarity.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures


References
-
- Bretschneider T., Diez S., Anderson K., Heuser J., Clarke M., Müller-Taubenberger A., Köhler J., Gerisch G. Curr. Biol. 2004;14:1–10. - PubMed
-
- Westphal M., Jungbluth A., Heidecker M., Mühlbauer B., Heizer C., Schwartz J., Marriott G., Gerisch G. Curr. Biol. 1997;7:176–183. - PubMed
-
- Pruyne D., Bretscher A. J. Cell Sci. 2000;113:571–585. - PubMed
-
- Møller-Jensen J., Borch J., Dam M., Jensen R. B., Roepstorff P., Gerdes K. Mol. Cell. 2003;12:1477–1487. - PubMed
-
- Soufo H. J. D., Graumann P. L. Curr. Biol. 2003;13:1916–1920. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

