Desensitization of GSTF8 induction by a prior chemical treatment is long lasting and operates in a tissue-dependent manner
- PMID: 16829588
- PMCID: PMC1557611
- DOI: 10.1104/pp.106.079509
Desensitization of GSTF8 induction by a prior chemical treatment is long lasting and operates in a tissue-dependent manner
Abstract
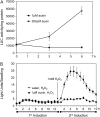
The Arabidopsis (Arabidopsis thaliana) GSTF8 gene is a member of the glutathione S-transferase (GST) family whose expression is induced by defense signals, certain chemical stresses, and some pathogens. Here, we have used transgenic plants and an in vivo imaging system to demonstrate that GSTF8 expression is subject to a distinct desensitization phenomenon because prior chemical treatment significantly reduces reactivation of the GSTF8 promoter by hydrogen peroxide, auxin, and salicylic acid. A GSTF8 null line had similar desensitization properties to wild type, demonstrating that GSTF8 protein levels are not responsible for desensitization. The resulting refractory period is unusually long lasting, with full recovery taking 4 d. Expression of the GSTF8 promoter following a second treatment occurred predominantly in newly formed tissue at the root tip, suggesting that desensitization is lost upon cell division. Expression of the endogenous GSTF8 gene and another GST gene, GSTF6, is also desensitized following treatment with hydrogen peroxide. The desensitization phenomenon can be activated by a very low concentration of inducer that is not sufficient to activate the GSTF8 promoter. These results demonstrate that activation of the GSTF8 promoter is not essential for eliciting desensitization. A key promoter sequence within the GSTF8 gene, the ocs element, is also affected by desensitization. Treatment with a phosphatase inhibitor prevents desensitization of GSTF8 expression and ocs element activity, suggesting that dephosphorylation of one or more proteins is required for desensitization to occur.
Figures






Similar articles
-
Early induction of the Arabidopsis GSTF8 promoter by specific strains of the fungal pathogen Rhizoctonia solani.Mol Plant Microbe Interact. 2004 Jan;17(1):70-80. doi: 10.1094/MPMI.2004.17.1.70. Mol Plant Microbe Interact. 2004. PMID: 14714870
-
Differential gene expression and subcellular targeting of Arabidopsis glutathione S-transferase F8 is achieved through alternative transcription start sites.J Biol Chem. 2007 Sep 28;282(39):28915-28928. doi: 10.1074/jbc.M702207200. Epub 2007 Jul 31. J Biol Chem. 2007. PMID: 17670748
-
TGA5 acts as a positive and TGA4 acts as a negative regulator of ocs element activity in Arabidopsis roots in response to defence signals.FEBS Lett. 2004 Apr 9;563(1-3):141-5. doi: 10.1016/S0014-5793(04)00288-1. FEBS Lett. 2004. PMID: 15063738
-
The auxin, hydrogen peroxide and salicylic acid induced expression of the Arabidopsis GST6 promoter is mediated in part by an ocs element.Plant J. 1999 Sep;19(6):667-77. doi: 10.1046/j.1365-313x.1999.00560.x. Plant J. 1999. PMID: 10571852
-
The promoter of a H2O2-inducible, Arabidopsis glutathione S-transferase gene contains closely linked OBF- and OBP1-binding sites.Plant J. 1996 Dec;10(6):955-66. doi: 10.1046/j.1365-313x.1996.10060955.x. Plant J. 1996. PMID: 9011080
Cited by
-
Glutathione transferases.Arabidopsis Book. 2010;8:e0131. doi: 10.1199/tab.0131. Epub 2010 May 8. Arabidopsis Book. 2010. PMID: 22303257 Free PMC article.
-
A low-temperature-responsive element involved in the regulation of the Arabidopsis thaliana At1g71850/At1g71860 divergent gene pair.Plant Cell Rep. 2016 Aug;35(8):1757-67. doi: 10.1007/s00299-016-1994-y. Epub 2016 May 23. Plant Cell Rep. 2016. PMID: 27215439
-
Glutathione-indole-3-acetonitrile is required for camalexin biosynthesis in Arabidopsis thaliana.Plant Cell. 2011 Jan;23(1):364-80. doi: 10.1105/tpc.110.079145. Epub 2011 Jan 14. Plant Cell. 2011. PMID: 21239642 Free PMC article.
-
The Arabidopsis altered in stress response2 is Impaired in Resistance to Root and Leaf Necrotrophic Fungal Pathogens.Plants (Basel). 2019 Mar 11;8(3):60. doi: 10.3390/plants8030060. Plants (Basel). 2019. PMID: 30862010 Free PMC article.
-
The Arabidopsis RNA Polymerase II Carboxyl Terminal Domain (CTD) Phosphatase-Like1 (CPL1) is a biotic stress susceptibility gene.Sci Rep. 2018 Sep 7;8(1):13454. doi: 10.1038/s41598-018-31837-0. Sci Rep. 2018. PMID: 30194343 Free PMC article.
References
-
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 - PubMed
-
- Armitage JP (1992) Behavioral responses in bacteria. Annu Rev Physiol 54: 683–714 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

