Simian immunodeficiency virus (SIV)/immunoglobulin G immune complexes in SIV-infected macaques block detection of CD16 but not cytolytic activity of natural killer cells
- PMID: 16829614
- PMCID: PMC1489573
- DOI: 10.1128/CVI.00042-06
Simian immunodeficiency virus (SIV)/immunoglobulin G immune complexes in SIV-infected macaques block detection of CD16 but not cytolytic activity of natural killer cells
Abstract
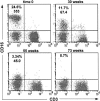
Natural killer cells are components of the innate immune system that play an important role in eliminating viruses and malignant cells. Using simian immunodeficiency virus (SIV) infection of macaques as a model, flow cytometry revealed a gradual loss of CD16+ NK cell numbers that was associated with disease progression. Of note, the apparent loss of NK cells was detected in whole-blood samples but not in isolated peripheral blood mononuclear cells (PBMC), suggesting that an inhibitor(s) of the antibody used to detect CD16, the low-affinity immunoglobulin G (IgG) receptor, was present in blood but was removed during PBMC isolation. (Actual decreases in CD16+ cell numbers in PBMC generally were not detected until animals became lymphopenic.) The putative decrease in CD16+ cell numbers in whole blood correlated with increasing SIV-specific antibody titers and levels of plasma virion RNA. With the addition of increasing amounts of plasma from progressor, but not nonprogressor, macaques to PBMC from an uninfected animal, the apparent percentage of CD16+ cells and the mean fluorescence intensity of antibodies binding to CD16 declined proportionately. A similar decrease was observed with the addition of monomeric IgG (mIgG) and IgG immune complexes (IgG-ICs) purified from the inhibitory plasma samples; some of the ICs contained SIV p27(gag) antigen and/or virions. Of interest, addition of purified IgG/IgG-ICs to NK cell lytic assays did not inhibit killing of K562 cells. These results indicate that during progressive SIV and, by inference, human immunodeficiency virus disease, CD16+ NK cell numbers can be underestimated, or the cells not detected at all, when one is using a whole-blood fluorescence-activated cell sorter assay and a fluorochrome-labeled antibody that can be blocked by mIgG or IgG-ICs. Although this blocking had no apparent effect on NK cell activity in vitro, the in vivo effects are unknown.
Figures




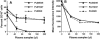

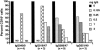
Similar articles
-
Functional analyses of natural killer cells in macaques infected with neurovirulent simian immunodeficiency virus.J Neurovirol. 2001 Feb;7(1):11-24. doi: 10.1080/135502801300069593. J Neurovirol. 2001. PMID: 11519478
-
Accumulation of Cytotoxic CD16+ NK Cells in Simian Immunodeficiency Virus-Infected Lymph Nodes Associated with In Situ Differentiation and Functional Anergy.J Virol. 2015 Jul;89(13):6887-94. doi: 10.1128/JVI.00660-15. Epub 2015 Apr 22. J Virol. 2015. PMID: 25903330 Free PMC article.
-
Passive immune globulin therapy in the SIV/macaque model: early intervention can alter disease profile.Immunol Lett. 1996 Jun;51(1-2):107-14. doi: 10.1016/0165-2478(96)02563-1. Immunol Lett. 1996. PMID: 8811353
-
Antibody-dependent cellular cytotoxicity in HIV infection.AIDS. 2018 Nov 13;32(17):2439-2451. doi: 10.1097/QAD.0000000000002011. AIDS. 2018. PMID: 30234611 Free PMC article. Review.
-
Innate immune cell responses in non pathogenic versus pathogenic SIV infections.Curr Opin Virol. 2016 Aug;19:37-44. doi: 10.1016/j.coviro.2016.06.011. Epub 2016 Jul 20. Curr Opin Virol. 2016. PMID: 27447445 Review.
Cited by
-
Robust NK cell-mediated human immunodeficiency virus (HIV)-specific antibody-dependent responses in HIV-infected subjects.J Virol. 2008 Jun;82(11):5450-9. doi: 10.1128/JVI.01952-07. Epub 2008 Mar 19. J Virol. 2008. PMID: 18353957 Free PMC article.
-
Monocyte heterogeneity underlying phenotypic changes in monocytes according to SIV disease stage.J Leukoc Biol. 2010 Apr;87(4):557-67. doi: 10.1189/jlb.0209082. Epub 2009 Oct 20. J Leukoc Biol. 2010. PMID: 19843579 Free PMC article.
-
Systemic dendritic cell mobilization associated with administration of FLT3 ligand to SIV- and SHIV-infected macaques.AIDS Res Hum Retroviruses. 2009 Dec;25(12):1313-28. doi: 10.1089/aid.2009.0053. AIDS Res Hum Retroviruses. 2009. PMID: 20001520 Free PMC article.
-
Use of an anti-CD16 antibody for in vivo depletion of natural killer cells in rhesus macaques.Immunology. 2008 Jun;124(2):215-22. doi: 10.1111/j.1365-2567.2007.02757.x. Epub 2008 Jan 12. Immunology. 2008. PMID: 18201184 Free PMC article.
-
CD16- natural killer cells: enrichment in mucosal and secondary lymphoid tissues and altered function during chronic SIV infection.Blood. 2010 Jun 3;115(22):4439-46. doi: 10.1182/blood-2010-01-265595. Epub 2010 Mar 25. Blood. 2010. PMID: 20339088 Free PMC article.
References
-
- Alter, G., J. M. Malenfant, R. M. Delabre, N. C. Burgett, X. G. Yu, M. Lichterfeld, J. Zaunders, and M. Altfeld. 2004. Increased natural killer cell activity in viremic HIV-1 infection. J. Immunol. 173:5305-5311. - PubMed
-
- Azzoni, L., E. Papasavvas, J. Chehimi, J. R. Kostman, K. Mounzer, J. Ondercin, B. Perussia, and L. J. Montaner. 2002. Sustained impairment of IFN-γ secretion in suppressed HIV-infected patients despite mature NK cell recovery: evidence for a defective reconstitution of innate immunity. J. Immunol. 168:5764-5770. - PubMed
-
- Azzoni, L., R. M. Rutstein, J. Chehimi, M. A. Farabaugh, A. Nowmos, and L. J. Montaner. 2005. Dendritic and natural killer cell subsets associated with stable or declining CD4+ cell counts in treated HIV-1-infected children. J. Infect. Dis. 191:1451-1459. - PubMed
-
- Banks, N. D., N. Kinsey, J. Clements, and J. E. K. Hildreth. 2002. Sustained antibody-dependent cell-mediated cytotoxicity (ADCC) in SIV-infected macaques correlates with delayed progression to AIDS. AIDS Res. Hum. Retrovir. 18:1197-1205. - PubMed
-
- Baum, L. L., K. J. Cassutt, K. Knigge, R. Khattri, J. Margolick, C. Rinaldo, C. A. Kleeberger, P. Nishanian, D. R. Henrard, and J. Phair. 1996. HIV-1 gp120-specific antibody-dependent cell-mediated cytotoxicity correlates with rate of disease progression. J. Immunol. 157:2168-2173. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

