Understanding the immune responses to the meningococcal strain-specific vaccine MeNZB measured in studies of infants
- PMID: 16829618
- PMCID: PMC1489567
- DOI: 10.1128/CVI.00038-06
Understanding the immune responses to the meningococcal strain-specific vaccine MeNZB measured in studies of infants
Abstract
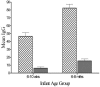
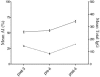
Vaccine trials with infants enrolled between 6 and 10 weeks of age (young infants) and 6 and 8 months of age (older infants) provided an opportunity to evaluate immunoglobulin G (IgG) isotype distribution and avidity maturation as indicators of antibody function and immunologic memory. Following vaccination with a strain-specific outer membrane vaccine, MeNZB, pre- and postvaccination sera were used to determine IgG isotype responses and avidity indices (AI) in subsets of vaccinated subjects. Measurements of IgG isotypes involved 100 infants from each trial. AI were measured in 50 infants from the young infant trial who received a fourth vaccine dose and in 40 older infants from whom serum was collected 7 months after the primary vaccination course. IgG1 and IgG3 dominated the responses to the vaccine. A modest linear correlation (P < 0.001) occurred between serum bactericidal antibody (SBAb) titers and the total IgG or the IgG1 antibody units in older infants. The young infants showed a modest linear correlation between SBAb and total IgG (P = 0.005) and a weak linear correlation between SBAb and IgG1 (P = 0.003). Increased avidity with age was demonstrated in both groups. The AI in the young infants increased from 51.5% (95% confidence interval [CI], 47.7 to 54.7) postvaccination to 68.7% (95% CI, 65.5 to 71.9%) following the fourth dose of vaccine (P < 0.001). The mean avidity of the older infants increased significantly (P = 0.00012) from 42.4% (95% CI, 39.1 to 45.3%) postvaccination to 50.4% (95% CI, 47.2 to 53.6%) 4 months later. A fourth dose of MeNZB is now being given to young infants at 10 months of age.
Figures
Similar articles
-
Antibody avidity and immunoglobulin G isotype distribution following immunization with a monovalent meningococcal B outer membrane vesicle vaccine.Infect Immun. 2002 Feb;70(2):584-90. doi: 10.1128/IAI.70.2.584-590.2002. Infect Immun. 2002. PMID: 11796586 Free PMC article. Clinical Trial.
-
Antibody persistence following MeNZB vaccination of adults and children and response to a fourth dose in toddlers.Arch Dis Child. 2011 Aug;96(8):744-51. doi: 10.1136/adc.2009.180596. Epub 2011 May 19. Arch Dis Child. 2011. PMID: 21596727 Clinical Trial.
-
Immunogenicity and tolerability in infants of a New Zealand epidemic strain meningococcal B outer membrane vesicle vaccine.Pediatr Infect Dis J. 2009 May;28(5):385-90. doi: 10.1097/INF.0b013e318195205e. Pediatr Infect Dis J. 2009. PMID: 19384263 Clinical Trial.
-
Immunogenicity and safety of a combination of two serogroup B meningococcal outer membrane vesicle vaccines.Clin Vaccine Immunol. 2007 Sep;14(9):1062-9. doi: 10.1128/CVI.00094-07. Epub 2007 Jul 18. Clin Vaccine Immunol. 2007. PMID: 17634513 Free PMC article. Clinical Trial.
-
Fast tracking the vaccine licensure process to control an epidemic of serogroup B meningococcal disease in New Zealand.Clin Infect Dis. 2009 Aug 15;49(4):597-605. doi: 10.1086/603552. Clin Infect Dis. 2009. PMID: 19622040 Review.
Cited by
-
Functional and specific antibody responses in adult volunteers in new zealand who were given one of two different meningococcal serogroup B outer membrane vesicle vaccines.Clin Vaccine Immunol. 2007 Jul;14(7):830-8. doi: 10.1128/CVI.00039-07. Epub 2007 May 9. Clin Vaccine Immunol. 2007. PMID: 17494638 Free PMC article. Clinical Trial.
-
Neisseria meningitidis native outer membrane vesicles containing different lipopolysaccharide glycoforms as adjuvants for meningococcal and nonmeningococcal antigens.Clin Vaccine Immunol. 2014 Feb;21(2):234-42. doi: 10.1128/CVI.00561-13. Epub 2013 Dec 18. Clin Vaccine Immunol. 2014. PMID: 24351756 Free PMC article.
-
Diversity of Greek meningococcal serogroup B isolates and estimated coverage of the 4CMenB meningococcal vaccine.BMC Microbiol. 2014 Apr 29;14:111. doi: 10.1186/1471-2180-14-111. BMC Microbiol. 2014. PMID: 24779381 Free PMC article.
-
Role of factor H binding protein in Neisseria meningitidis virulence and its potential as a vaccine candidate to broadly protect against meningococcal disease.Microbiol Mol Biol Rev. 2013 Jun;77(2):234-52. doi: 10.1128/MMBR.00056-12. Microbiol Mol Biol Rev. 2013. PMID: 23699256 Free PMC article. Review.
-
Impact of meningococcal group B OMV vaccines, beyond their brief.Hum Vaccin Immunother. 2018 May 4;14(5):1058-1063. doi: 10.1080/21645515.2017.1381810. Epub 2017 Nov 17. Hum Vaccin Immunother. 2018. PMID: 29048985 Free PMC article.
References
-
- Aase, A., E. A. Hoiby, and T. E. Michaelsen. 1998. Opsonophagocytic and bactericidal activity mediated by purified IgG subclass antibodies after vaccination with the Norwegian group B meningococcal vaccine. Scand. J. Immunol. 47:388-396. - PubMed
-
- Aase, A., G. Bjune, E. A. Hoiby, E. Rosenqvist, A. K. Pedersen, and T. E. Michaelsen. 1995. Comparison among opsonic activity, antimeningococcal immunoglobulin G response, and serum bactericidal activity against meningococci in sera from vaccinees after immunization with a serogroup B outer membrane vesicle vaccine. Infect. Immun. 63:3531-3536. - PMC - PubMed
-
- de Kleijn, E., L. van Eijndhoven, C. Vermont, B. Kuipers, H. van Dijken, H. Rumke, R. de Groot, L. van Alphen, and G. van der Dobbelsteen. 2001. Serum bactericidal activity and isotype distribution of antibodies in toddlers and schoolchildren after vaccination with RIVM hexavalent PorA vesicle vaccine. Vaccine 20:352-358. - PubMed
-
- Dyet, K., A. Devoy, R. McDowell, and D. Martin. 2005. New Zealand's epidemic of meningococcal disease described using molecular analysis: implications for vaccine delivery. Vaccine 23:2228-2230. - PubMed
-
- Garred, P., T. E. Michaelsen, and A. Aase. 1989. The IgG subclass pattern of complement activation depends on epitope density and antibody and complement concentration. Scand. J. Immunol. 30:379-382. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

