The biogenesis and regulation of telomerase holoenzymes
- PMID: 16829980
- PMCID: PMC2915765
- DOI: 10.1038/nrm1961
The biogenesis and regulation of telomerase holoenzymes
Abstract
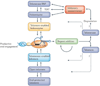
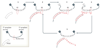
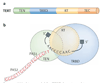
Chromosome stability requires a dynamic balance of DNA loss and gain in each terminal tract of telomeric repeats. Repeat addition by a specialized reverse transcriptase, telomerase, has an important role in maintaining this equilibrium. Insights that have been gained into the cellular pathways for biogenesis and regulation of telomerase ribonucleoproteins raise new questions, particularly concerning the dynamic nature of this unique polymerase.
Figures

References
-
- Blackburn EH, Gall JG. A tandemly repeated sequence at the termini of the extrachromosomal ribosomal RNA genes in Tetrahymena. J. Mol. Biol. 1978;120:33–53. - PubMed
-
- de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19:2100–2110. - PubMed
-
- Bhattacharyya MK, Lustig AJ. Telomere dynamics in genome stability. Trends Biochem. Sci. 2006;31:114–122. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

