Successful induction of clinically competent dendritic cells from granulocyte colony-stimulating factor-mobilized monocytes for cancer vaccine therapy
- PMID: 16830156
- PMCID: PMC11030097
- DOI: 10.1007/s00262-006-0197-8
Successful induction of clinically competent dendritic cells from granulocyte colony-stimulating factor-mobilized monocytes for cancer vaccine therapy
Abstract
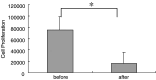
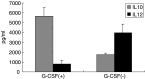
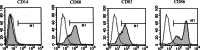
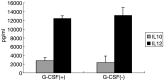
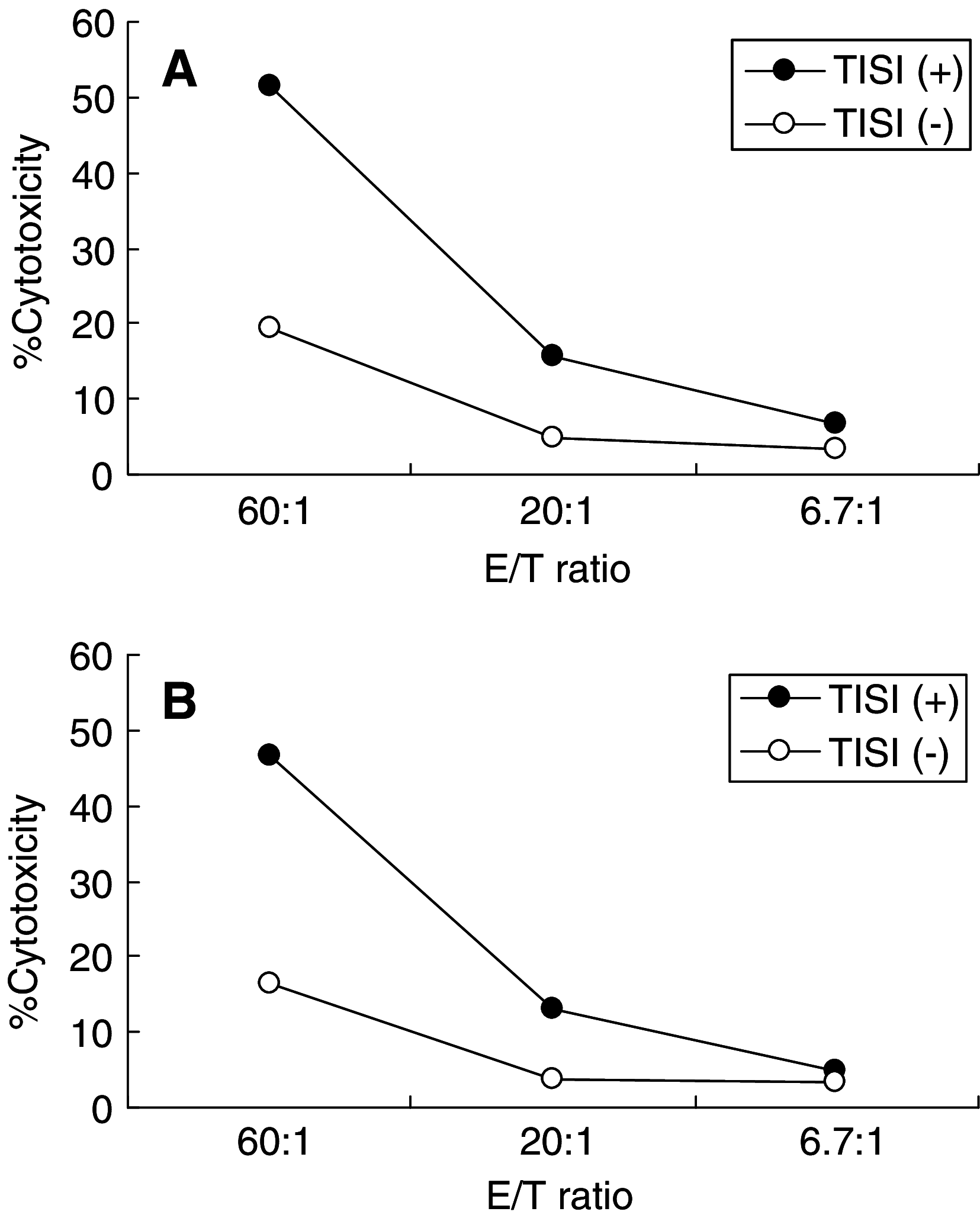
Recent studies have suggested that dendritic cell (DC)-based immunotherapy is one promising approach for the treatment of cancer. We previously studied the clinical toxicity, feasibility, and efficacy of cancer vaccine therapy with peptide-pulsed DCs. In that study, we used granulocyte colony-stimulating factor (G-CSF)-mobilized peripheral blood monocytes as a cell source of DCs. However, previous investigations have suggested that G-CSF-mobilized peripheral blood monocytes produce reduced levels of proinflammatory cytokines such as interleukin (IL)-12 and tumor necrosis factor (TNF)-alpha. These T helper (Th)-1-type cytokines are thought to promote antitumor immune response. In this study, we assessed the functional abilities of DCs generated from G-CSF-mobilized monocytes obtained from 13 patients with CEA-positive advanced solid cancers. Peripheral blood mononuclear cells were obtained from leukapheresis products collected before and after systemic administration of G-CSF (subcutaneous administration of high-dose [5-10 microg/kg] human recombinant G-CSF for five consecutive days). In vitro cytokine production profiles after stimulation with lipopolysaccharide (LPS) were compared between monocytes with and without G-CSF mobilization. DCs generated from monocytes were also examined with respect to cytokine production and the capacity to induce peptide-specific T cell responses. Administration of G-CSF was found to efficiently mobilize peripheral blood monocytes. Although G-CSF-mobilized monocytes (G/Mo) less effectively produced Th-1-type cytokines than control monocytes (C/Mo), DCs generated from G/Mo restored the same level of IL-12 production as that seen in DCs generated from C/Mo. T cell induction assay using recall antigen peptide and phenotypic analyses also demonstrated that DCs generated from G/Mo retained characteristics identical to those generated from C/Mo. Our results suggest that G-CSF mobilization can be used to collect monocytes as a cell source for the generation of DCs for cancer immunotherapy. DCs generated in this fashion were pulsed with HLA-A24-restricted CEA epitope peptide and administered to patients safely; immunological responses were induced in some patients.
Figures

References
-
- Schuler TB, Schultz ES, Berger TG, Weinlich G, Ebner S, Woerl P, et al. Rapid induction of tumor-specific type 1 T helper cells in metastatic melanoma patients by vaccination with mature, cryopreserved, peptide-loaded monocyte-derived dendritic cells. J Exp Med. 2002;195:1279. doi: 10.1084/jem.20012100. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

