Gut hormones, and short bowel syndrome: the enigmatic role of glucagon-like peptide-2 in the regulation of intestinal adaptation
- PMID: 16830359
- PMCID: PMC4087358
- DOI: 10.3748/wjg.v12.i26.4117
Gut hormones, and short bowel syndrome: the enigmatic role of glucagon-like peptide-2 in the regulation of intestinal adaptation
Abstract
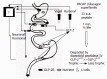
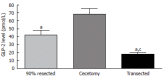
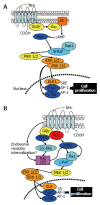
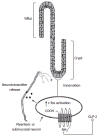
Short bowel syndrome (SBS) refers to the malabsorption of nutrients, water, and essential vitamins as a result of disease or surgical removal of parts of the small intestine. The most common reasons for removing part of the small intestine are due to surgical intervention for the treatment of either Crohn's disease or necrotizing enterocolitis. Intestinal adaptation following resection may take weeks to months to be achieved, thus nutritional support requires a variety of therapeutic measures, which include parenteral nutrition. Improper nutrition management can leave the SBS patient malnourished and/or dehydrated, which can be life threatening. The development of therapeutic strategies that reduce both the complications and medical costs associated with SBS/long-term parenteral nutrition while enhancing the intestinal adaptive response would be valuable. Currently, therapeutic options available for the treatment of SBS are limited. There are many potential stimulators of intestinal adaptation including peptide hormones, growth factors, and neuronally-derived components. Glucagon-like peptide-2 (GLP-2) is one potential treatment for gastrointestinal disorders associated with insufficient mucosal function. A significant body of evidence demonstrates that GLP-2 is a trophic hormone that plays an important role in controlling intestinal adaptation. Recent data from clinical trials demonstrate that GLP-2 is safe, well-tolerated, and promotes intestinal growth in SBS patients. However, the mechanism of action and the localization of the glucagon-like peptide-2 receptor (GLP-2R) remains an enigma. This review summarizes the role of a number of mucosal-derived factors that might be involved with intestinal adaptation processes; however, this discussion primarily examines the physiology, mechanism of action, and utility of GLP-2 in the regulation of intestinal mucosal growth.
Figures
Similar articles
-
Short bowel syndrome: the role of GLP-2 on improving outcome.Curr Opin Clin Nutr Metab Care. 2009 Sep;12(5):526-32. doi: 10.1097/MCO.0b013e32832d23cd. Curr Opin Clin Nutr Metab Care. 2009. PMID: 19474717 Review.
-
Glucagon-like peptide-2 induces a specific pattern of adaptation in remnant jejunum.Dig Dis Sci. 2006 Sep;51(9):1557-66. doi: 10.1007/s10620-006-9077-5. Epub 2006 Aug 22. Dig Dis Sci. 2006. PMID: 16927140
-
Glucagon-like peptide-2 induces rapid digestive adaptation following intestinal resection in preterm neonates.Am J Physiol Gastrointest Liver Physiol. 2013 Aug 15;305(4):G277-85. doi: 10.1152/ajpgi.00064.2013. Epub 2013 Jun 13. Am J Physiol Gastrointest Liver Physiol. 2013. PMID: 23764891 Free PMC article.
-
Effects of exogenous glucagon-like peptide-2 and distal bowel resection on intestinal and systemic adaptive responses in rats.PLoS One. 2017 Jul 24;12(7):e0181453. doi: 10.1371/journal.pone.0181453. eCollection 2017. PLoS One. 2017. PMID: 28738080 Free PMC article.
-
Short bowel syndrome in children: current and potential therapies.Paediatr Drugs. 2012 Jun 1;14(3):179-88. doi: 10.2165/11594880-000000000-00000. Paediatr Drugs. 2012. PMID: 22452596 Review.
Cited by
-
Modulation of mouse intestinal epithelial cell turnover in the absence of angiotensin converting enzyme.Am J Physiol Gastrointest Liver Physiol. 2008 Jul;295(1):G88-G98. doi: 10.1152/ajpgi.00589.2007. Epub 2008 May 15. Am J Physiol Gastrointest Liver Physiol. 2008. PMID: 18483182 Free PMC article.
-
Regulation of body weight: Lessons learned from bariatric surgery.Mol Metab. 2023 Feb;68:101517. doi: 10.1016/j.molmet.2022.101517. Epub 2022 May 26. Mol Metab. 2023. PMID: 35644477 Free PMC article. Review.
-
Administration of a dipeptidyl peptidase IV inhibitor enhances the intestinal adaptation in a mouse model of short bowel syndrome.Surgery. 2011 Aug;150(2):217-23. doi: 10.1016/j.surg.2011.05.013. Epub 2011 Jun 30. Surgery. 2011. PMID: 21719060 Free PMC article.
-
A pilot study examining the relationship among Crohn disease activity, glucagon-like peptide-2 signalling and intestinal function in pediatric patients.Can J Gastroenterol. 2013 Oct;27(10):587-92. doi: 10.1155/2013/460958. Can J Gastroenterol. 2013. PMID: 24106731 Free PMC article.
-
The real-world analysis of adverse events with teduglutide: a pharmacovigilance study based on the FAERS database.Front Pharmacol. 2024 Sep 12;15:1404658. doi: 10.3389/fphar.2024.1404658. eCollection 2024. Front Pharmacol. 2024. PMID: 39329127 Free PMC article.
References
-
- Dowling RH, Booth CC. Structural and functional changes following small intestinal resection in the rat. Clin Sci. 1967;32:139–149. - PubMed
-
- Vanderhoof JA. Short bowel syndrome in children and small intestinal transplantation. Pediatr Clin North Am. 1996;43:533–550. - PubMed
-
- Williamson RC. Intestinal adaptation (first of two parts). Structural, functional and cytokinetic changes. N Engl J Med. 1978;298:1393–1402. - PubMed
-
- Sigalet DL. Short bowel syndrome in infants and children: an overview. Semin Pediatr Surg. 2001;10:49–55. - PubMed
-
- Vanderhoof JA, Langnas AN, Pinch LW, Thompson JS, Kaufman SS. Short bowel syndrome. J Pediatr Gastroenterol Nutr. 1992;14:359–370. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

