CCR2 mediates increases in glial activation caused by exposure to HIV-1 Tat and opiates
- PMID: 16831471
- PMCID: PMC4310703
- DOI: 10.1016/j.jneuroim.2006.05.027
CCR2 mediates increases in glial activation caused by exposure to HIV-1 Tat and opiates
Abstract
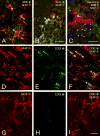
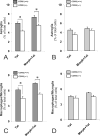
To assess the role of CCL2/MCP-1 in opiate drug abuse and HIV-1 comorbidity, the effects of systemic morphine and intrastriatal HIV-1 Tat on macrophage/microglial and astroglial activation were assessed in wild type and CCR2 null mice. Tat and/or morphine additively increased the proportion of CCL2 immunoreactive astroglia. The effects of morphine were prevented by naltrexone. Glial activation was significantly reduced in CCR2-/- versus wild-type mice following Tat or morphine plus Tat exposure. Thus, CCR2 contributes to local glial activation caused by Tat alone or in the presence of opiates, implicating CCR2 signaling in HIV-1 neuropathogenesis in drug abusers and non-abusers.
Figures
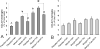
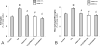
References
-
- Ambati J, Anand A, Fernandez S, Sakurai E, Lynn BC, Kuziel WA, Rollins BJ, Ambati BK. An animal model of age-related macular degeneration in senescent Ccl-2- or Ccr-2-deficient mice. Nat Med. 2003;9:1390–1397. - PubMed
-
- Andjelkovic AV, Kerkovich D, Shanley J, Pulliam L, Pachter JS. Expression of binding sites for beta chemokines on human astrocytes. Glia. 1999;28:225–235. - PubMed
-
- Andjelkovic AV, Song L, Dzenko KA, Cong H, Pachter JS. Functional expression of CCR2 by human fetal astrocytes. J Neurosci Res. 2002;70:219–231. - PubMed
-
- Avison MJ, Nath A, Greene-Avison R, Schmitt FA, Bales RA, Ethisham A, Greenberg RN, Berger JR. Inflammatory changes and breakdown of microvascular integrity in early human immunodeficiency virus dementia. J Neurovirol. 2004;10:223–232. - PubMed
-
- Banisadr G, Queraud-Lesaux F, Boutterin MC, Pelaprat D, Zalc B, Rostene W, Haour F, Parsadaniantz SM. Distribution, cellular localization and functional role of CCR2 chemokine receptors in adult rat brain. J Neurochem. 2002;81:257–269. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

