BDNF mobilizes synaptic vesicles and enhances synapse formation by disrupting cadherin-beta-catenin interactions
- PMID: 16831887
- PMCID: PMC2064188
- DOI: 10.1083/jcb.200601087
BDNF mobilizes synaptic vesicles and enhances synapse formation by disrupting cadherin-beta-catenin interactions
Abstract
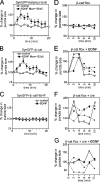
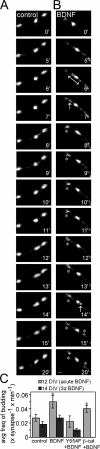
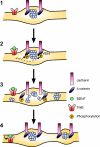
Neurons of the vertebrate central nervous system have the capacity to modify synapse number, morphology, and efficacy in response to activity. Some of these functions can be attributed to activity-induced synthesis and secretion of the neurotrophin brain-derived neurotrophic factor (BDNF); however, the molecular mechanisms by which BDNF mediates these events are still not well understood. Using time-lapse confocal analysis, we show that BDNF mobilizes synaptic vesicles at existing synapses, resulting in small clusters of synaptic vesicles "splitting" away from synaptic sites. We demonstrate that BDNF's ability to mobilize synaptic vesicle clusters depends on the dissociation of cadherin-beta-catenin adhesion complexes that occurs after tyrosine phosphorylation of beta-catenin. Artificially maintaining cadherin-beta-catenin complexes in the presence of BDNF abolishes the BDNF-mediated enhancement of synaptic vesicle mobility, as well as the longer-term BDNF-mediated increase in synapse number. Together, this data demonstrates that the disruption of cadherin-beta-catenin complexes is an important molecular event through which BDNF increases synapse density in cultured hippocampal neurons.
Figures



References
-
- Ahmari, S.E., J. Buchanan, and S.J. Smith. 2000. Assembly of presynaptic active zones from cytoplasmic transport packets. Nat. Neurosci. 3:445–451. - PubMed
-
- Applegate, M.D., D.S. Kerr, and P.W. Landfield. 1987. Redistribution of synaptic vesicles during long-term potentiation in the hippocampus. Brain Res. 401:401–406. - PubMed
-
- Bamji, S.X. 2005. Cadherins: actin with the cytoskeleton to form synapses. Neuron. 47:175–178. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

