The NF-kappaB regulator Bcl-3 and the BH3-only proteins Bim and Puma control the death of activated T cells
- PMID: 16832056
- PMCID: PMC1544160
- DOI: 10.1073/pnas.0603625103
The NF-kappaB regulator Bcl-3 and the BH3-only proteins Bim and Puma control the death of activated T cells
Abstract
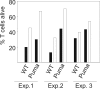
Apoptosis of activated T cells is critical for the termination of immune responses. Here we show that adjuvant-stimulated dendritic cells secrete cytokines that prime activated T cells for survival and analyze the roles of the NF-kappaB regulator Bcl-3 and the proapoptotic Bcl-2 family members Bim and Puma. Bcl-3 overexpression increased survival, and activated bcl-3-/- T cells died abnormally rapidly. Cytokines from adjuvant-stimulated dendritic cells induced Bcl-3, but survival through cytokine priming was Bcl-3-independent. Apoptosis inhibition by Bcl-3 involved blockade of Bim activation, because Bim was overactivated in Bcl-3-deficient cells, and Bcl-3 failed to increase survival of bim-/- T cells. However, adjuvants increased survival also in Bim-deficient T cells. This Bim-independent death pathway is at least in part regulated by Puma, as shown by analysis of puma-/- and noxa-/- T cells. IL-1, IL-7, and IL-15 primed T cells for survival even in the absence of Bim or Puma. Our data define interrelations and a Bim-independent pathway to activated T cell death.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
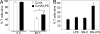




Similar articles
-
BH3-only proteins Puma and Bim are rate-limiting for gamma-radiation- and glucocorticoid-induced apoptosis of lymphoid cells in vivo.Blood. 2005 Dec 15;106(13):4131-8. doi: 10.1182/blood-2005-04-1595. Epub 2005 Aug 23. Blood. 2005. PMID: 16118324 Free PMC article.
-
The proapoptotic BH3-only proteins Bim and Puma are downstream of endoplasmic reticulum and mitochondrial oxidative stress in pancreatic islets in response to glucotoxicity.Cell Death Dis. 2014 Mar 13;5(3):e1124. doi: 10.1038/cddis.2014.88. Cell Death Dis. 2014. PMID: 24625983 Free PMC article.
-
Puma cooperates with Bim, the rate-limiting BH3-only protein in cell death during lymphocyte development, in apoptosis induction.J Exp Med. 2006 Dec 25;203(13):2939-51. doi: 10.1084/jem.20061552. Epub 2006 Dec 18. J Exp Med. 2006. PMID: 17178918 Free PMC article.
-
Apoptosis in activated T cells: what are the triggers, and what the signal transducers?Cell Cycle. 2006 Nov 1;5(21):2421-4. doi: 10.4161/cc.5.21.3397. Epub 2006 Sep 12. Cell Cycle. 2006. PMID: 17102629 Review.
-
Role of Bim and other Bcl-2 family members in autoimmune and degenerative diseases.Curr Dir Autoimmun. 2006;9:74-94. doi: 10.1159/000090773. Curr Dir Autoimmun. 2006. PMID: 16394656 Review.
Cited by
-
High glucose induces apoptosis via upregulation of Bim expression in proximal tubule epithelial cells.Oncotarget. 2017 Apr 11;8(15):24119-24129. doi: 10.18632/oncotarget.15491. Oncotarget. 2017. PMID: 28445931 Free PMC article.
-
Gene expression network analysis reveals new transcriptional regulators as novel factors in human ischemic cardiomyopathy.BMC Med Genomics. 2015 Mar 29;8:14. doi: 10.1186/s12920-015-0088-y. BMC Med Genomics. 2015. PMID: 25884818 Free PMC article.
-
Loss of the pro-apoptotic BH3-only Bcl-2 family member Bim sustains B lymphopoiesis in the absence of IL-7.Int Immunol. 2009 Jun;21(6):715-25. doi: 10.1093/intimm/dxp043. Epub 2009 May 19. Int Immunol. 2009. PMID: 19454543 Free PMC article.
-
PUMA-mediated intestinal epithelial apoptosis contributes to ulcerative colitis in humans and mice.J Clin Invest. 2011 May;121(5):1722-32. doi: 10.1172/JCI42917. Epub 2011 Apr 1. J Clin Invest. 2011. PMID: 21490394 Free PMC article.
-
Transgenic Bcl-3 slows T cell proliferation.Int Immunol. 2009 Apr;21(4):339-48. doi: 10.1093/intimm/dxp002. Epub 2009 Feb 10. Int Immunol. 2009. PMID: 19208752 Free PMC article.
References
-
- Strasser A. Nat. Rev. Immunol. 2005;5:189–200. - PubMed
-
- Marrack P., Kappler J. Annu. Rev. Immunol. 2004;22:765–787. - PubMed
-
- Hildeman D. A., Zhu Y., Mitchell T. C., Bouillet P., Strasser A., Kappler J., Marrack P. Immunity. 2002;16:759–767. - PubMed
-
- Adams J. M. Genes Dev. 2003;17:2481–2495. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

