Essential role of nitric oxide in VEGF-induced, asthma-like angiogenic, inflammatory, mucus, and physiologic responses in the lung
- PMID: 16832062
- PMCID: PMC1544167
- DOI: 10.1073/pnas.0601057103
Essential role of nitric oxide in VEGF-induced, asthma-like angiogenic, inflammatory, mucus, and physiologic responses in the lung
Abstract
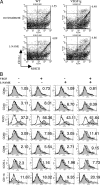
VEGF, nitric oxide (NO), inflammation, and vascular- and extravascular remodeling coexist in asthma and other disorders. In these responses, VEGF regulates angiogenesis. VEGF also induces inflammation and remodeling. The mechanisms of the latter responses have not been defined, however. We hypothesized that VEGF-induces extravascular tissue responses via NO-dependent mechanisms. To evaluate this hypothesis, we compared the effects of transgenic VEGF165 in lungs from normal mice, mice treated with pan-NO synthase (NOS) or endothelial NOS (eNOS) inhibitors, and mice with null mutations of inducible NOS (iNOS) or eNOS. These studies demonstrate that VEGF selectively stimulates eNOS and iNOS. They also demonstrate that VEGF induces pulmonary alterations via NO-dependent and -independent mechanisms with angiogenesis, edema, mucus metaplasia, airway hyperresponsiveness, lymphocyte accumulation, dendritic cell hyperplasia and S-nitrosoglutathione reductase stimulation being NO-dependent and dendritic cell activation being NO-independent. Furthermore, they demonstrate that eNOS and iNOS both contribute to these responses. NO/NOS-based interventions may be therapeutic in VEGF-driven inflammation and remodeling.
Figures

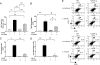


References
-
- Tammela T., Enholm B., Alitalo K., Paavonen K. Cardiovasc. Res. 2005;65:550–563. - PubMed
-
- Ferrara N., Gerber H. P., LeCouter J. Nat. Med. 2003;9:669–676. - PubMed
-
- Reynolds L. P., Redmer D. A. Biol. Reprod. 2001;64:1033–1040. - PubMed
-
- Silha J. V., Krsek M., Sucharda P., Murphy L. J. Int. J. Obes. Relat. Metab. Disord. 2005 - PubMed
-
- Khurana R., Simons M., Martin J. F., Zachary I. C. Circulation. 2005;112:1813–1824. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

