Evaluation of biologically relevant short alpha-helices stabilized by a main-chain hydrogen-bond surrogate
- PMID: 16834399
- PMCID: PMC1828873
- DOI: 10.1021/ja062710w
Evaluation of biologically relevant short alpha-helices stabilized by a main-chain hydrogen-bond surrogate
Abstract
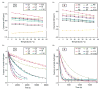
We previously reported the design and synthesis of a new class of artificial alpha-helices in which an N-terminal main-chain hydrogen bond is replaced by a carbon-carbon bond derived from a ring-closing metathesis reaction [Chapman, R. N.; Dimartino, G.; Arora, P. S. J. Am. Chem. Soc. 2004, 126, 12252-12253]. Our initial study utilized an alanine-rich sequence; in the present manuscript we evaluate the potential of this method for the synthesis of very short (10 residues) alpha-helices representing two different biologically relevant alpha-helical domains. We extensively characterized these two sets of artificial helices by NMR and circular dichroism spectroscopies and find that the hydrogen-bond surrogate approach can afford well-defined short alpha-helical structures from sequences that do not spontaneously form alpha-helical conformations.
Figures
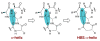






References
-
- Shepherd NE, Hoang HN, Abbenante G, Fairlie DP. J Am Chem Soc. 2005;127:2974. - PubMed
-
- Lifson S, Roig A. J Chem Phys. 1961;34:1963.
-
- Zimm BH, Bragg JK. J Chem Phys. 1959;31:526.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

