Effects of highly active antiretroviral therapy with nelfinavir in vertically HIV-1 infected children: 3 years of follow-up. Long-term response to nelfinavir in children
- PMID: 16834769
- PMCID: PMC1538605
- DOI: 10.1186/1471-2334-6-107
Effects of highly active antiretroviral therapy with nelfinavir in vertically HIV-1 infected children: 3 years of follow-up. Long-term response to nelfinavir in children
Abstract
Background: Antiretroviral treatment (ART) in children has special features and consequently, results obtained from clinical trials with antiretroviral drugs in adults may not be representative of children. Nelfinavir (NFV) is an HIV-1 Protease Inhibitor (PI) which has become as one of the first choices of PI for ART in children. We studied during a 3-year follow-up period the effects of highly active antiretroviral therapy with nelfinavir in vertically HIV-1 infected children.
Methods: Forty-two vertically HIV-infected children on HAART with NFV were involved in a multicentre prospective study. The children were monitored at least every 3 months with physical examinations, and blood sample collection to measure viral load (VL) and CD4+ cell count. We performed a logistic regression analysis to determinate the odds ratio of baseline characteristics on therapeutic failure.
Results: Very important increase in CD4+ was observed and VL decreased quickly and it remained low during the follow-up study. Children with CD4+ <25% at baseline achieved CD4+ >25% at 9 months of follow-up. HIV-infected children who achieved undetectable viral load (uVL) were less than 40% in each visit during follow-up. Nevertheless, HIV-infected children with VL >5000 copies/ml were less than 50% during the follow-up study. Only baseline VL was an important factor to predict VL control during follow-up. Virological failure at defined end-point was confirmed in 30/42 patients. Along the whole of follow-up, 16/42 children stopped HAART with NFV. Baseline characteristics were not associated with therapeutic change.
Conclusion: NFV is a safe drug with a good profile and able to achieve an adequate response in children.
Figures
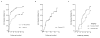
Similar articles
-
Long-term response to highly active antiretroviral therapy with lopinavir/ritonavir in pre-treated vertically HIV-infected children.J Antimicrob Chemother. 2008 Jan;61(1):183-90. doi: 10.1093/jac/dkm436. Epub 2007 Nov 19. J Antimicrob Chemother. 2008. PMID: 18025025
-
Effect of antiretroviral triple combinations including the protease inhibitor nelfinavir in heavily pretreated children with HIV-1 infection.Eur J Med Res. 2002 Jul 24;7(7):330-4. Eur J Med Res. 2002. PMID: 12176683
-
Long-term effects of highly active antiretroviral therapy in pretreated, vertically HIV type 1-infected children: 6 years of follow-up.Clin Infect Dis. 2006 Mar 15;42(6):862-9. doi: 10.1086/500412. Epub 2006 Feb 9. Clin Infect Dis. 2006. PMID: 16477566
-
[Recommendations from the GESIDA/Spanish AIDS Plan regarding antiretroviral treatment in adults with human immunodeficiency virus infection (update February 2009)].Enferm Infecc Microbiol Clin. 2009 Apr;27(4):222-35. doi: 10.1016/j.eimc.2008.11.002. Epub 2009 Feb 26. Enferm Infecc Microbiol Clin. 2009. PMID: 19246124 Spanish.
-
[Revised guideline "Antiretroviral Treatment"].Ned Tijdschr Geneeskd. 2005 Oct 22;149(43):2399-405. Ned Tijdschr Geneeskd. 2005. PMID: 16277129 Review. Dutch.
Cited by
-
Pharmacotherapy of pediatric and adolescent HIV infection.Ther Clin Risk Manag. 2009 Jun;5(3):469-84. doi: 10.2147/tcrm.s4594. Epub 2009 Jun 22. Ther Clin Risk Manag. 2009. PMID: 19707256 Free PMC article.
-
New diagnoses of human immunodeficiency virus infection in the Spanish pediatric HIV Cohort (CoRISpe) from 2004 to 2013.Medicine (Baltimore). 2017 Sep;96(39):e7858. doi: 10.1097/MD.0000000000007858. Medicine (Baltimore). 2017. PMID: 28953612 Free PMC article.
References
-
- de Martino M, Tovo PA, Balducci M, Galli L, Gabiano C, Rezza G, Pezzotti P. Reduction in mortality with availability of antiretroviral therapy for children with perinatal HIV-1 infection. Italian Register for HIV Infection in Children and the Italian National AIDS Registry. JAMA. 2000;284:190–197. doi: 10.1001/jama.284.2.190. - DOI - PubMed
-
- Resino S, Bellón JM, Resino R, Navarro ML, Ramos JT, Mellado MJ, de Jose MI, Muñoz-Fernández MA. Extensive implementation of highly active antiretroviral therapy shows great effectiveness on the survival and surrogate markers in vertically HIV-infected children. Clin Infect Dis. 2004;38:1605–1612. doi: 10.1086/420738. - DOI - PubMed
-
- Krogstad P, Lee S, Johnson G, Stanley K, McNamara J, Moye J, Jackson JB, Aguayo R, Dieudonne A, Khoury M, Mendez H, Nachman S, Wiznia A. Nucleoside-analogue reverse-transcriptase inhibitors plus nevirapine, nelfinavir, or ritonavir for pretreated children infected with human immunodeficiency virus type 1. Clin Infect Dis. 2002;34:991–1001. doi: 10.1086/338814. - DOI - PubMed
-
- Gatti G, Castelli-Gattinara G, Cruciani M, Bernardi S, De Pascalis CR, Pontali E, Papa L, Miletich F, Bassetti D. Pharmacokinetics and pharmacodynamics of nelfinavir administered twice or thrice daily to human immunodeficiency virus type 1-infected children. Clin Infect Dis. 2003;36:1476–1482. doi: 10.1086/375062. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

