The active metabolite of Clopidogrel disrupts P2Y12 receptor oligomers and partitions them out of lipid rafts
- PMID: 16835302
- PMCID: PMC1635153
- DOI: 10.1073/pnas.0510446103
The active metabolite of Clopidogrel disrupts P2Y12 receptor oligomers and partitions them out of lipid rafts
Abstract
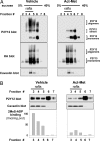
P2Y12, a G protein-coupled receptor that plays a central role in platelet activation has been recently identified as the receptor targeted by the antithrombotic drug, clopidogrel. In this study, we further deciphered the mechanism of action of clopidogrel and of its active metabolite (Act-Met) on P2Y12 receptors. Using biochemical approaches, we demonstrated the existence of homooligomeric complexes of P2Y12 receptors at the surface of mammalian cells and in freshly isolated platelets. In vitro treatment with Act-Met or in vivo oral administration to rats with clopidogrel induced the breakdown of these oligomers into dimeric and monomeric entities in P2Y12 expressing HEK293 and platelets respectively. In addition, we showed the predominant association of P2Y12 oligomers to cell membrane lipid rafts and the partitioning of P2Y12 out of rafts in response to clopidogrel and Act-Met. The raft-associated P2Y12 oligomers represented the functional form of the receptor, as demonstrated by binding and signal transduction studies. Finally, using a series of receptors individually mutated at each cysteine residue and a chimeric P2Y12/P2Y13 receptor, we pointed out the involvement of cysteine 97 within the first extracellular loop of P2Y12 in the mechanism of action of Act-Met.
Conflict of interest statement
Conflict of interest statement: P.S., J.-L.Z., N.D.-T., C.L., C.H., M.-F.U., J.-M.P., J.-M.C., F.B., P.F., and J.-M.H. are employees of Sanofi-Aventis.
Figures


Similar articles
-
P2Y12, a new platelet ADP receptor, target of clopidogrel.Semin Vasc Med. 2003 May;3(2):113-22. doi: 10.1055/s-2003-40669. Semin Vasc Med. 2003. PMID: 15199474 Review.
-
Molecular identification and characterization of the platelet ADP receptor targeted by thienopyridine antithrombotic drugs.J Clin Invest. 2001 Jun;107(12):1591-8. doi: 10.1172/JCI12242. J Clin Invest. 2001. PMID: 11413167 Free PMC article.
-
Platelet aggregometry and receptor binding to predict the magnitude of antithrombotic and bleeding time effects of clopidogrel in rabbits.J Cardiovasc Pharmacol. 2007 May;49(5):316-24. doi: 10.1097/FJC.0b013e31803e8772. J Cardiovasc Pharmacol. 2007. PMID: 17513951
-
Molecular modeling of purinergic receptor P2Y12 and interaction with its antagonists.J Mol Graph Model. 2007 Jul;26(1):20-31. doi: 10.1016/j.jmgm.2006.09.006. Epub 2006 Sep 26. J Mol Graph Model. 2007. PMID: 17110146
-
Bleeding manifestations of congenital and drug-induced defects of the platelet P2Y12 receptor for adenosine diphosphate.Thromb Haemost. 2011 May;105 Suppl 1:S67-74. doi: 10.1160/THS10-11-0742. Epub 2011 Apr 11. Thromb Haemost. 2011. PMID: 21479342 Review.
Cited by
-
The effectiveness and safety of triple-antiplatelet treatment based on cilostazol for patients receiving percutaneous coronary intervention: a meta-analysis.Clin Cardiol. 2012 Oct;35(10):598-604. doi: 10.1002/clc.22001. Epub 2012 May 14. Clin Cardiol. 2012. PMID: 22585740 Free PMC article. Review.
-
P2Y receptors in the mammalian nervous system: pharmacology, ligands and therapeutic potential.CNS Neurol Disord Drug Targets. 2012 Sep;11(6):722-38. doi: 10.2174/187152712803581047. CNS Neurol Disord Drug Targets. 2012. PMID: 22963441 Free PMC article. Review.
-
IFN-stimulated P2Y13 protects mice from viral infection by suppressing the cAMP/EPAC1 signaling pathway.J Mol Cell Biol. 2019 May 1;11(5):395-407. doi: 10.1093/jmcb/mjy045. J Mol Cell Biol. 2019. PMID: 30137373 Free PMC article.
-
Association of serum levels of lipoprotein A-I and lipoprotein A-I/A-II with high on-treatment platelet reactivity in patients with ST-segment elevation myocardial infarction.Anatol J Cardiol. 2018 Jun;19(6):374-381. doi: 10.14744/AnatolJCardiol.2018.63549. Anatol J Cardiol. 2018. PMID: 29848921 Free PMC article.
-
Arrestin-2 differentially regulates PAR4 and ADP receptor signaling in platelets.J Biol Chem. 2011 Feb 4;286(5):3805-14. doi: 10.1074/jbc.M110.118018. Epub 2010 Nov 24. J Biol Chem. 2011. PMID: 21106537 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

