Plasmodium falciparum AMA1 binds a rhoptry neck protein homologous to TgRON4, a component of the moving junction in Toxoplasma gondii
- PMID: 16835460
- PMCID: PMC1489286
- DOI: 10.1128/EC.00040-06
Plasmodium falciparum AMA1 binds a rhoptry neck protein homologous to TgRON4, a component of the moving junction in Toxoplasma gondii
Abstract
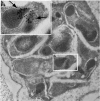
Plasmodium falciparum apical membrane antigen 1 (PfAMA1) coimmunoprecipitates with the Plasmodium homologue of TgRON4, a secreted rhoptry neck protein of Toxoplasma gondii that migrates at the moving junction in association with TgAMA1 during invasion. PfRON4 also originates in the rhoptry necks, suggesting that this unusual collaboration of micronemes and rhoptries is a conserved feature of Apicomplexa.
Figures

References
-
- Bannister, L. H., J. M. Hopkins, A. R. Dluzewski, G. Margos, I. T. Williams, M. J. Blackman, C. H. Kocken, A. W. Thomas, and G. H. Mitchell. 2003. Plasmodium falciparum apical membrane antigen 1 (PfAMA-1) is translocated within micronemes along subpellicular microtubules during merozoite development. J. Cell Sci. 116:3825-3834. - PubMed
-
- Blackman, M. J. 1994. Purification of Plasmodium falciparum merozoites for analysis of the processing of merozoite surface protein-1. Methods Cell Biol. 45:213-220. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

