Elevation of soluble interleukin-2 receptor in patients with non-small cell lung cancer treated with gefitinib
- PMID: 16835747
- PMCID: PMC12161027
- DOI: 10.1007/s00432-006-0120-x
Elevation of soluble interleukin-2 receptor in patients with non-small cell lung cancer treated with gefitinib
Abstract
Purpose: We previously reported that plasma thromboxan B(2), soluble P-selectin, and serum regulated on activation, normal T-cell expressed and secreted (RANTES) were elevated after gefitinib treatment. We hypothesized that gefitinib could activate T-lymphocytes via activated platelets, and so we measured serum levels of soluble interleukin-2 receptor (sIL-2R) in patients medicated with gefitinib.
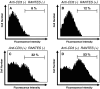
Methods: Twenty-one patients with non-small cell lung cancer (NSCLC) were entered into this study. All patients received gefitinib over 2 weeks without severe adverse effects. Blood samples were withdrawn from all patients before and after the administration of gefitinib and plasma soluble P-selectin, serum RANTES, and serum sIL-2R were measured by enzyme-linked immunosolvent assay. In addition, we carried out the basic study of the interleukin-2 receptor (IL-2R) expression on CD4(+) lymphocytes by RANTES.
Results: Plasma soluble P-selectin, serum RANTES, and serum sIL-2R levels increased significantly in patients receiving gefitinib treatment for 1 and 2 weeks. RANTES did not induce the expression of IL-2R on CD4(+) lymphocyte. However, the anti-CD3 monoclonal antibody-induced expression of IL-2R was enhanced by the addition of RANTES.
Conclusion: Our finding indicated that lymphocytes were activated by gefitinib treatment. We think that sIL-2R elevation after gefitinib administration may be a factor positively effecting patients with NSCLC. It is deemed possible that the effect of gefitinib is induced not only by its blocking of the tyrosine kinase of epidermal growth factor receptor but also by antitumor immunity via its activation of T-cells.
Figures


Similar articles
-
First-line treatment of advanced epidermal growth factor receptor (EGFR) mutation positive non-squamous non-small cell lung cancer.Cochrane Database Syst Rev. 2016 May 25;(5):CD010383. doi: 10.1002/14651858.CD010383.pub2. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2021 Mar 18;3:CD010383. doi: 10.1002/14651858.CD010383.pub3. PMID: 27223332 Updated.
-
Gefitinib for advanced non-small cell lung cancer.Cochrane Database Syst Rev. 2018 Jan 16;1(1):CD006847. doi: 10.1002/14651858.CD006847.pub2. Cochrane Database Syst Rev. 2018. PMID: 29336009 Free PMC article.
-
Prediction of epidermal growth factor receptor mutations in the plasma/pleural effusion to efficacy of gefitinib treatment in advanced non-small cell lung cancer.J Cancer Res Clin Oncol. 2010 Sep;136(9):1341-7. doi: 10.1007/s00432-010-0785-z. Epub 2010 Feb 14. J Cancer Res Clin Oncol. 2010. PMID: 20155428 Free PMC article.
-
Erlotinib and gefitinib for treating non-small cell lung cancer that has progressed following prior chemotherapy (review of NICE technology appraisals 162 and 175): a systematic review and economic evaluation.Health Technol Assess. 2015 Jun;19(47):1-134. doi: 10.3310/hta19470. Health Technol Assess. 2015. PMID: 26134145 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
Cited by
-
Skin inflammatory response and efficacy of anti-epidermal growth factor receptor therapy in metastatic colorectal cancer (CUTACETUX).Oncoimmunology. 2020 Nov 29;9(1):1848058. doi: 10.1080/2162402X.2020.1848058. Oncoimmunology. 2020. PMID: 33299659 Free PMC article.
-
Serum sCD25 Protein as a Predictor of Lack of Long-Term Benefits from Immunotherapy in Non-Small Cell Lung Cancer: A Pilot Study.Cancers (Basel). 2021 Jul 23;13(15):3702. doi: 10.3390/cancers13153702. Cancers (Basel). 2021. PMID: 34359602 Free PMC article.
-
Identification of Key Genes and Pathways in Gefitinib-Resistant Lung Adenocarcinoma using Bioinformatics Analysis.Evol Bioinform Online. 2021 Jun 11;17:11769343211023767. doi: 10.1177/11769343211023767. eCollection 2021. Evol Bioinform Online. 2021. PMID: 34177255 Free PMC article.
-
The Impact of Immunological Checkpoint Inhibitors and Targeted Therapy on Chronic Pruritus in Cancer Patients.Biomedicines. 2020 Dec 22;9(1):2. doi: 10.3390/biomedicines9010002. Biomedicines. 2020. PMID: 33375183 Free PMC article. Review.
-
The Predicting Role of Serum TSGF and sIL-2R for the Lymph Node Metastasis of Papillary Thyroid Carcinoma.Dis Markers. 2022 Sep 2;2022:3730679. doi: 10.1155/2022/3730679. eCollection 2022. Dis Markers. 2022. PMID: 36092957 Free PMC article.
References
-
- Ciardiello F, Tortora G (2001) A novel approach in the treatment of cancer: targeting the epidermal growth factor receptor. Clin Cancer Res 7:2958–2970 - PubMed
-
- Frasci G, Panza P, Comella P, Nicolella GP, Natale M, Pacilo C, Gravina A, Caputi V, Botti G, Comella G (1997) Cisplatin, gemcitabine, and vinorelbine in locally advanced or metastatic non-small-cell lung cancer: a phase I study. Ann Oncol 8:1045–1048 - PubMed
-
- Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Douillard JY, Nishiwaki Y, Vansteenkiste J, Kudoh S, Rischin D, Fek R, Horai T, Noda K, Takata I, Smit E, Averbuch S, Macleod A, Feyereilova A, Dong RP, Baselga J (2003) Multi institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer. J Clin Oncol 21:2237–2246 - PubMed
-
- Henderson BE, Ross RK, Pike MC (1991) Toward the primary prevention of cancer. Science 254:1131–1137 - PubMed
-
- Herbst RS, Giaccone G, Schiller JH, Natale RB, Miller V, Manegold C, Scagliotti G, Rosell R, Oliff I, Reeves JA, Wolf MK, Krebs AD, Averbuch SD, Ochs JS, Grous J, Fandi A, Johnson DH (2004) Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial—INTACT 2. J Clin Oncol 22:785–794 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

