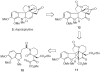
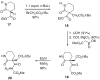
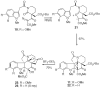
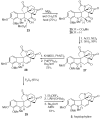
Application of the Rh(II) cyclization/cycloaddition cascade for the total synthesis of (+/-)-aspidophytine
- PMID: 16836384
- PMCID: PMC2604816
- DOI: 10.1021/ol061137i
Application of the Rh(II) cyclization/cycloaddition cascade for the total synthesis of (+/-)-aspidophytine
Abstract
[Structure: see text] A new strategy for the synthesis of (+/-)-aspidophytine has been developed and is based on a Rh(II)-catalyzed cyclization/dipolar cycloaddition sequence. The resulting [3+2]-cycloadduct undergoes an efficient Lewis acid mediated cascade that rapidly provides the complete skeleton of aspidophytine. The synthesis also features a mild decarbomethoxylation reaction.
Figures
Similar articles
-
General entry to aspidosperma alkaloids: enantioselective total synthesis of (-)-aspidophytine.Angew Chem Int Ed Engl. 2013 Jun 3;52(23):6015-8. doi: 10.1002/anie.201302442. Epub 2013 Apr 22. Angew Chem Int Ed Engl. 2013. PMID: 23610074
-
Enantioselective total synthesis of aspidophytine.Org Lett. 2003 May 29;5(11):1891-3. doi: 10.1021/ol034445e. Org Lett. 2003. PMID: 12762679
-
Enantioselective palladium-catalyzed allylic alkylation reactions in the synthesis of Aspidosperma and structurally related monoterpene indole alkaloids.Nat Prod Rep. 2018 Jun 20;35(6):559-574. doi: 10.1039/c7np00069c. Nat Prod Rep. 2018. PMID: 29658039 Free PMC article. Review.
-
Enantioselective Catalysis Coupled with Stereodivergent Cyclization Strategies Enables Rapid Syntheses of (+)-Limaspermidine and (+)-Kopsihainanine A.Angew Chem Int Ed Engl. 2017 Oct 2;56(41):12624-12627. doi: 10.1002/anie.201707304. Epub 2017 Sep 5. Angew Chem Int Ed Engl. 2017. PMID: 28872739 Free PMC article.
-
Advances and Strategies towards Synthesis of Aspidosperma Indole Alkaloids Goniomitine.Chem Biodivers. 2024 Jun;21(6):e202400416. doi: 10.1002/cbdv.202400416. Epub 2024 May 1. Chem Biodivers. 2024. PMID: 38587971 Review.
Cited by
-
Concise Total Syntheses of (+)-Haplocidine and (+)-Haplocine via Late-Stage Oxidation of (+)-Fendleridine Derivatives.J Am Chem Soc. 2016 Sep 7;138(35):11383-9. doi: 10.1021/jacs.6b07623. Epub 2016 Aug 26. J Am Chem Soc. 2016. PMID: 27510728 Free PMC article.
-
Transition metal catalyzed [6 + 2] cycloadditions.RSC Adv. 2019 Aug 15;9(44):25554-25568. doi: 10.1039/c9ra02839k. eCollection 2019 Aug 13. RSC Adv. 2019. PMID: 35530050 Free PMC article. Review.
-
Cycloaddition protocol for the assembly of the hexacyclic framework associated with the kopsifoline alkaloids.Org Lett. 2006 Oct 26;8(22):5141-4. doi: 10.1021/ol062029z. Org Lett. 2006. PMID: 17048863 Free PMC article.
-
The Domino Way to Heterocycles.Tetrahedron. 2007 Jun 18;63(25):5341-5378. doi: 10.1016/j.tet.2007.03.158. Tetrahedron. 2007. PMID: 17940591 Free PMC article.
-
Synthesis of (+/-)-3H-epivincamine via a Rh(II)-triggered cyclization/cycloaddition cascade.Org Lett. 2007 Aug 16;9(17):3249-52. doi: 10.1021/ol071173x. Epub 2007 Jul 21. Org Lett. 2007. PMID: 17658832 Free PMC article.
References
-
- Saxton JE. In: The Alkaloids: Chemistry and Biology. Cordell GA, editor. Vol. 51. Academic Press; San Diego, CA: 1998. pp. 1–197.
-
-
Saxton JE. Indoles, Part 4: The Monoterpenoid Indole Alkaloids. Wiley; Chichester: 1983. Herbert RB. In: The Monoterpenoid Indole Alkaloids. Saxton JE, editor. Chapter 1 Wiley; Chichester: 1994. Supplement to Vol 25, part 4 of The Chemistry of Heterocyclic Compounds Toyota M, Ihara M. Nat Prod Rep. 1998:327. and references cited therein
-
-
-
For the first synthesis of aspidospermine and vindoline, see: Stork G, Dolfini JE. J Am Chem Soc. 1963;85:2872.Ando M, Buchi G, Ohnuma T. J Am Chem Soc. 1975;97:6880.
-
-
-
For some select methods to synthesize the pentacyclic framework of aspidospermidine (1), see: Camerman A, Camerman N, Kutney JP, Piers E, Trotter J. Tetrahedron Lett. 1965:637.Harley-Mason J, Kaplan M. J Chem Soc Chem Commun. 1967:915.Laronze J-Y, Laronze-Fontaine J, Lévy J, Le Men J. Tetrahedron Lett. 1974:491.Ban Y, Yoshida K, Goto J, Oishi T. J Am Chem Soc. 1981;103:6990.Gallagher T, Magnus P, Huffman J. J Am Chem Soc. 1982;104:1140.Wenkert E, Hudlicky T. J Org Chem. 1988;53:1953.Mandal SB, Giri VS, Sabeena MS, Pakrashi SC. J Org Chem. 1988;53:4236.Meyers AI, Berney D. J Org Chem. 1989;54:4673.Node M, Nagasawa H, Fugi K. J Org Chem. 1990;55:517.Le Menez P, Kunesch N, Lui S, Wenkert E. J Org Chem. 1991;56:2915.Desmaële D, d’Angelo J. J Org Chem. 1994;59:2292.Wenkert E, Lui S. J Org Chem. 1994;59:7677.Forns P, Diez A, Rubiralta M. J Org Chem. 1996;61:7882.Schultz AG, Pettus L. J Org Chem. 1997;62:6855.Callaghan O, Lampard C, Kennedy AR, Murphy JA. J Chem Soc Perkin Trans 1. 1999:995.Iyengar R, Schildknegt K, Aubé J. Org Lett. 2000;2:1625.Toczko MA, Heathcock CH. J Org Chem. 2000;65:2642.Patro B, Murphy JA. Org Lett. 2000;2:3599.Kozmin SA, Iwama T, Huang Y, Rawal VH. J Am Chem Soc. 2002;124:4628.Banwell MG, Smith JA. J Chem Soc Perkin Trans 1. 2002:2613.Marino JP, Rubio MB, Cao G, de Dios A. J Am Chem Soc. 2002;124:13398.Gnecco D, Vázquez E, Galindo A, Terán JL, Bernès S, Enríquez RG. Arkivoc. 2003;11:185.Tanino H, Fukuishi T, Ushiyama M, Okada K. Tetrahedron. 2004;60:3273.Banwell MG, Lupton DW. Org Biomol Chem. 2005;3:213.
-
-
- Yates P, MacLachlan FN, Rae ID, Rosenberger M, Szabo AG, Willis CR, Cava MP, Behforouz M, Lakshmikantham MV, Ziegler W. J Am Chem Soc. 1973;95:7842.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources