Photochemical treatment of plasma with amotosalen and long-wavelength ultraviolet light inactivates pathogens while retaining coagulation function
- PMID: 16836564
- PMCID: PMC7201872
- DOI: 10.1111/j.1537-2995.2006.00867.x
Photochemical treatment of plasma with amotosalen and long-wavelength ultraviolet light inactivates pathogens while retaining coagulation function
Abstract
Background: The INTERCEPT Blood System, a photochemical treatment (PCT) process, has been developed to inactivate pathogens in platelet concentrates. These studies evaluated the efficacy of PCT to inactivate pathogens in plasma and the effect of PCT on plasma function.
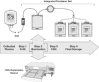
Study design and methods: Jumbo (600 mL) plasma units were inoculated with high titers of test pathogens and treated with 150 micromol per L amotosalen and 3 J per cm(2) long-wavelength ultraviolet light. The viability of each pathogen before and after treatment was measured with biological assays. Plasma function was evaluated through measurement of coagulation factors and antithrombotic protein activities.
Results: The levels of inactivation expressed as log-reduction were as follows: cell-free human immunodeficiency virus-1 (HIV-1), greater than 6.8; cell-associated HIV-1, greater than 6.4; human T-lymphotropic virus-I (HTLV-I), 4.5; HTLV-II, greater than 5.7; hepatitis B virus (HBV) and hepatitis C virus, greater than 4.5; duck HBV, 4.4 to 4.5; bovine viral diarrhea virus, 6.0; severe acute respiratory syndrome coronavirus, 5.5; West Nile virus, 6.8; bluetongue virus, 5.1; human adenovirus 5, 6.8; Klebsiella pneumoniae, greater than 7.4; Staphylococcus epidermidis and Yersinia enterocolitica, greater than 7.3; Treponema pallidum, greater than 5.9; Borrelia burgdorferi, greater than 10.6; Plasmodium falciparum, 6.9; Trypanosoma cruzi, greater than 5.0; and Babesia microti, greater than 5.3. Retention of coagulation factor activity after PCT was expressed as the proportion of pretreatment (baseline) activity. Retention was 72 to 73 percent of baseline fibrinogen and Factor (F)VIII activity and 78 to 98 percent for FII, FV, FVII, F IX, FX, FXI, FXIII, protein C, protein S, antithrombin, and alpha2-antiplasmin.
Conclusion: PCT of plasma inactivated high levels of a wide range of pathogens while maintaining adequate coagulation function. PCT has the potential to reduce the risk of transfusion-transmitted diseases in patients requiring plasma transfusion support.
Figures
References
-
- Sullivan MT, McCullough J, Schreiber GB, Wallace EL. Blood collection and transfusion in the United States in 1997. Transfusion 2002;42:1253‐60. - PubMed
-
- Practice Guidelines for blood component therapy: a report by the American Society of Anesthesiologists Task Force on Blood Component Therapy. Anesthesiology 1996;84:732‐47. - PubMed
-
- Dodd RY, Notari EP 4th, Stramer SL. Current prevalence and incidence of infectious disease markers and estimated window‐period risk in the American Red Cross blood donor population. Transfusion 2002;42:975‐9. - PubMed
-
- Barbara J. Why “safer than ever” may not be safe enough. Transfus Med Hemother 2004;31(Suppl 1):2‐10.
-
- Phelps R, Robbins K, Liberti T, et al. Window‐period human immunodeficiency virus transmission to two recipients by an adolescent blood donor. Transfusion 2004;44:929‐33. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

