Fab1 phosphatidylinositol 3-phosphate 5-kinase controls trafficking but not silencing of endocytosed receptors
- PMID: 16837550
- PMCID: PMC1556381
- DOI: 10.1091/mbc.e06-03-0239
Fab1 phosphatidylinositol 3-phosphate 5-kinase controls trafficking but not silencing of endocytosed receptors
Abstract
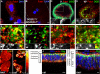
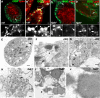
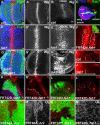
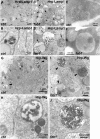
The trafficking of endocytosed receptors through phosphatidylinositol 3-phosphate [PtdIns(3)P]-containing endosomes is thought to attenuate their signaling. Here, we show that the PtdIns(3)P 5-kinase Fab1/PIKfyve controls trafficking but not silencing of endocytosed receptors. Drosophila fab1 mutants contain undetectable phosphatidylinositol 3,5-bisphosphate levels, show profound increases in cell and organ size, and die at the pupal stage. Mutant larvae contain highly enlarged multivesicular bodies and late endosomes that are inefficiently acidified. Clones of fab1 mutant cells accumulate Wingless and Notch, similarly to cells lacking Hrs, Vps25, and Tsg101, components of the endosomal sorting machinery for ubiquitinated membrane proteins. However, whereas hrs, vps25, and tsg101 mutant cell clones accumulate ubiquitinated cargo, this is not the case with fab1 mutants. Even though endocytic receptor trafficking is impaired in fab1 mutants, Notch, Wingless, and Dpp signaling is unaffected. We conclude that Fab1, despite its importance for endosomal functions, is not required for receptor silencing. This is consistent with the possibility that Fab1 functions at a late stage in endocytic receptor trafficking, at a point when signal termination has occurred.
Figures




References
-
- Aldaz S., Morata G., Azpiazu N. The Pax-homeobox gene eyegone is involved in the subdivision of the thorax of Drosophila. Development. 2003;130:4473–4482. - PubMed
-
- Basler K., Struhl G. Compartment boundaries and the control of Drosophila limb pattern by hedgehog protein. Nature. 1994;368:208–214. - PubMed
-
- Berwick D. C., Dell G. C., Welsh G. I., Heesom K. J., Hers I., Fletcher L. M., Cooke F. T., Tavare J. M. Protein kinase B phosphorylation of PIKfyve regulates the trafficking of GLUT4 vesicles. J. Cell Sci. 2004;117:5985–5993. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

