Neuronal calcium sensor-1 and phosphatidylinositol 4-kinase beta stimulate extracellular signal-regulated kinase 1/2 signaling by accelerating recycling through the endocytic recycling compartment
- PMID: 16837555
- PMCID: PMC1593177
- DOI: 10.1091/mbc.e05-11-1014
Neuronal calcium sensor-1 and phosphatidylinositol 4-kinase beta stimulate extracellular signal-regulated kinase 1/2 signaling by accelerating recycling through the endocytic recycling compartment
Abstract
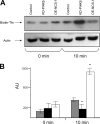
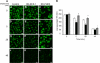
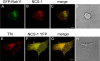
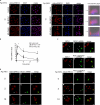
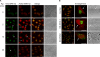
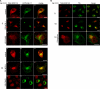
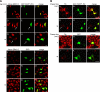
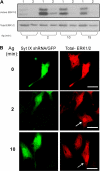
We demonstrate that recycling through the endocytic recycling compartment (ERC) is an essential step in Fc epsilonRI-induced activation of extracellular signal-regulated kinase (ERK)1/2. We show that ERK1/2 acquires perinuclear localization and colocalizes with Rab 11 and internalized transferrin in Fc epsilonRI-activated cells. Moreover, a close correlation exists between the amount of ERC-localized ERK1/2 and the amount of phospho-ERK1/2 that resides in the nucleus. We further show that by activating phosphatidylinositol 4-kinase beta (PI4Kbeta) and increasing the cellular level of phosphatidylinositol(4) phosphate, neuronal calcium sensor-1 (NCS-1), a calmodulin-related protein, stimulates recycling and thereby enhances Fc epsilonRI-triggered activation and nuclear translocation of ERK1/2. Conversely, NCS-1 short hairpin RNA, a kinase dead (KD) mutant of PI4Kbeta (KD-PI4Kbeta), the pleckstrin homology (PH) domain of FAPP1 as well as RNA interference of synaptotagmin IX or monensin, which inhibit export from the ERC, abrogate Fc epsilonRI-induced activation of ERK1/2. Consistently, NCS-1 also enhances, whereas both KD-PI4Kbeta and FAPP1-PH domain inhibit, Fc epsilonRI-induced release of arachidonic acid/metabolites, a downstream target of ERK1/2 in mast cells. Together, our results demonstrate a novel role for NCS-1 and PI4Kbeta in regulating ERK1/2 signaling and inflammatory reactions in mast cells. Our results further identify the ERC as a crucial determinant in controlling ERK1/2 signaling.
Figures

Similar articles
-
Neuronal calcium sensor-1 and phosphatidylinositol 4-kinase beta regulate IgE receptor-triggered exocytosis in cultured mast cells.J Immunol. 2003 Nov 15;171(10):5320-7. doi: 10.4049/jimmunol.171.10.5320. J Immunol. 2003. PMID: 14607934
-
The mast cell: where endocytosis and regulated exocytosis meet.Immunol Rev. 2007 Jun;217:292-303. doi: 10.1111/j.1600-065X.2007.00516.x. Immunol Rev. 2007. PMID: 17498067 Review.
-
Interaction of neuronal calcium sensor-1 (NCS-1) with phosphatidylinositol 4-kinase beta stimulates lipid kinase activity and affects membrane trafficking in COS-7 cells.J Biol Chem. 2001 Oct 26;276(43):40183-9. doi: 10.1074/jbc.M104048200. Epub 2001 Aug 28. J Biol Chem. 2001. PMID: 11526106
-
Neuronal calcium sensor 1 and phosphatidylinositol 4-OH kinase beta interact in neuronal cells and are translocated to membranes during nucleotide-evoked exocytosis.J Cell Sci. 2002 Oct 15;115(Pt 20):3909-22. doi: 10.1242/jcs.00072. J Cell Sci. 2002. PMID: 12244129
-
Role of myristoylation in the intracellular targeting of neuronal calcium sensor (NCS) proteins.Biochem Soc Trans. 2003 Oct;31(Pt 5):963-5. doi: 10.1042/bst0310963. Biochem Soc Trans. 2003. PMID: 14505460 Review.
Cited by
-
Phosphatidylinositol 4-kinases and PI4P metabolism in the nervous system: roles in psychiatric and neurological diseases.Mol Neurobiol. 2013 Feb;47(1):361-72. doi: 10.1007/s12035-012-8358-6. Epub 2012 Oct 10. Mol Neurobiol. 2013. PMID: 23054682 Review.
-
A dual role for cell plate-associated PI4Kβ in endocytosis and phragmoplast dynamics during plant somatic cytokinesis.EMBO J. 2019 Feb 15;38(4):e100303. doi: 10.15252/embj.2018100303. Epub 2019 Jan 7. EMBO J. 2019. PMID: 30617084 Free PMC article.
-
IL1-receptor accessory protein-like 1 (IL1RAPL1), a protein involved in cognitive functions, regulates N-type Ca2+-channel and neurite elongation.Proc Natl Acad Sci U S A. 2007 May 22;104(21):9063-8. doi: 10.1073/pnas.0701133104. Epub 2007 May 14. Proc Natl Acad Sci U S A. 2007. PMID: 17502602 Free PMC article.
-
Endosomal trafficking of the ligated FcvarepsilonRI receptor.Mol Immunol. 2009 Feb;46(5):793-802. doi: 10.1016/j.molimm.2008.09.002. Epub 2008 Oct 22. Mol Immunol. 2009. PMID: 18945491 Free PMC article.
-
The SNARE Machinery in Mast Cell Secretion.Front Immunol. 2012 Jun 5;3:143. doi: 10.3389/fimmu.2012.00143. eCollection 2012. Front Immunol. 2012. PMID: 22679448 Free PMC article.
References
-
- Birkeland H. C., Stenmark H. Protein targeting to endosomes and phagosomes via FYVE and PX domains. Curr. Top. Microbiol. Immunol. 2004;282:89–115. - PubMed
-
- Bourne Y., Dannenberg J., Pollmann V., Marchot P., Pongs O. Immunocytochemical localization and crystal structure of human frequenin (neuronal calcium sensor 1). J. Biol. Chem. 2001;276:11949–11955. - PubMed
-
- Braunewell K. H., Gundelfinger E. D. Intracellular neuronal calcium sensor proteins: a family of EF-hand calcium-binding proteins in search of a function. Cell Tissue Res. 1999;295:1–12. - PubMed
-
- Carpentier J. L., Dayer J. M., Lang U., Silverman R., Orci L., Gorden P. Down-regulation and recycling of insulin receptors. Effect of monensin on IM-9 lymphocytes and U-937 monocyte-like cells. J. Biol. Chem. 1984;259:14190–14195. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

