Role of the neurogranin concentrated in spines in the induction of long-term potentiation
- PMID: 16837580
- PMCID: PMC6674191
- DOI: 10.1523/JNEUROSCI.0729-06.2006
Role of the neurogranin concentrated in spines in the induction of long-term potentiation
Abstract
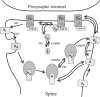
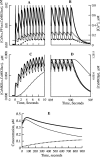
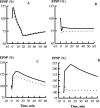
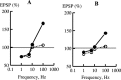
Synaptic plasticity in CA1 hippocampal neurons depends on Ca2+ elevation and the resulting activation of calmodulin-dependent enzymes. Induction of long-term depression (LTD) depends on calcineurin, whereas long-term potentiation (LTP) depends on Ca2+/calmodulin-dependent protein kinase II (CaMKII). The concentration of calmodulin in neurons is considerably less than the total concentration of the apocalmodulin-binding proteins neurogranin and GAP-43, resulting in a low level of free calmodulin in the resting state. Neurogranin is highly concentrated in dendritic spines. To elucidate the role of neurogranin in synaptic plasticity, we constructed a computational model with emphasis on the interaction of calmodulin with neurogranin, calcineurin, and CaMKII. The model shows how the Ca2+ transients that occur during LTD or LTP induction affect calmodulin and how the resulting activation of calcineurin and CaMKII affects AMPA receptor-mediated transmission. In the model, knockout of neurogranin strongly diminishes the LTP induced by a single 100 Hz, 1 s tetanus and slightly enhances LTD, in accord with experimental data. Our simulations show that exchange of calmodulin between a spine and its parent dendrite is limited. Therefore, inducing LTP with a short tetanus requires calmodulin stored in spines in the form of rapidly dissociating calmodulin-neurogranin complexes.
Figures



References
-
- Aigner L, Arber S, Kapfhammer JP, Laux T, Schneider C, Botteri F, Brenner HR, Caroni P (1995). Overexpression of the neural growth-associated protein Gap-43 induces nerve sprouting in the adult nervous system of transgenic mice. Cell 83:269–278. - PubMed
-
- Alexander KA, Cimler BM, Meier KE, Storm DR (1987). Regulation of calmodulin binding to P-57—a neurospecific calmodulin binding protein. J Biol Chem 262:6108–6113. - PubMed
-
- Baudier J, Deloulme JC, Vandorsselaer A, Black D, Matthes HWD (1991). Purification and characterization of a brain-specific protein kinase C substrate, neurogranin (P17)—identification of a consensus amino-acid sequence between neurogranin and neuromodulin (GAP43) that corresponds to the protein kinase C phosphorylation site and the calmodulin-binding domain. J Biol Chem 266:229–237. - PubMed
-
- Beattie EC, Carroll RC, Yu X, Morishita W, Yasuda H, von Zastrow M, Malenka RC (2000). Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nat Neurosci 3:1291–1300. - PubMed
-
- Bhalla US, Iyengar R (1999). Emergent properties of networks of biological signaling pathways. Science 283:381–387. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
