Influence of response variability on the coding performance of central gustatory neurons
- PMID: 16837591
- PMCID: PMC6674201
- DOI: 10.1523/JNEUROSCI.0106-06.2006
Influence of response variability on the coding performance of central gustatory neurons
Abstract
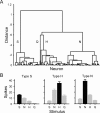
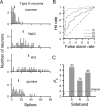
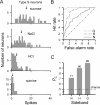
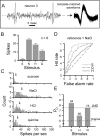
We explored how variability in responding to taste stimuli could impact the signaling of taste quality information by neuron types and individual cells in the nucleus of the solitary tract. One hundred sixty-two neurons recorded from anesthetized rats were grouped using multivariate analysis of taste responses to the following (in m): 0.5 sucrose, 0.1 NaCl, 0.01 HCl, and 0.01 quinine-HCl. Neurons fell into one of three groups corresponding to cell types that responded optimally to sucrose, NaCl, or HCl. A statistical model was used to examine whether responses observed among neurons within each group could be correctly attributed to the optimal stimulus or another tastant on the basis of spike count. Results revealed poor classification performance in some cases attributable to wide variations in the sensitivities of neurons that compose a cell type. This outcome leads us to question whether neuron types could faithfully encode a single taste quality. We then theoretically explored whether a hypothetical observer of individual neurons could discriminate between spiking rates to different tastants during the first second of stimulus processing. Spike rate was found to be an unreliable predictor of stimulus quality for each neuron tested. However, additional analyses suggested that taste stimuli could be identified by a reader that attends to the relative spiking activities of different kinds of neurons in parallel. Rather than assigning meaning to individual neurons or categories of them, central gustatory circuits may signal quality information using a strategy that involves the relative activities of neurons with different sensitivities to tastants.
Figures





Similar articles
-
Neural representation of bitter taste in the nucleus of the solitary tract.J Neurophysiol. 2005 Dec;94(6):3719-29. doi: 10.1152/jn.00700.2005. Epub 2005 Aug 17. J Neurophysiol. 2005. PMID: 16107527
-
Responses to binary taste mixtures in the nucleus of the solitary tract: neural coding with firing rate.J Neurophysiol. 2008 May;99(5):2144-57. doi: 10.1152/jn.01020.2007. Epub 2008 Feb 20. J Neurophysiol. 2008. PMID: 18287552
-
Variability in responses and temporal coding of tastants of similar quality in the nucleus of the solitary tract of the rat.J Neurophysiol. 2008 Feb;99(2):644-55. doi: 10.1152/jn.00920.2007. Epub 2007 Oct 3. J Neurophysiol. 2008. PMID: 17913985 Free PMC article.
-
Temporal coding of intensity of NaCl and HCl in the nucleus of the solitary tract of the rat.J Neurophysiol. 2011 Feb;105(2):697-711. doi: 10.1152/jn.00539.2010. Epub 2010 Nov 24. J Neurophysiol. 2011. PMID: 21106899 Free PMC article.
-
Cell types in the rostral nucleus of the solitary tract.Brain Res Brain Res Rev. 1995 Feb;20(2):185-95. doi: 10.1016/0165-0173(94)00011-d. Brain Res Brain Res Rev. 1995. PMID: 7795656 Review.
Cited by
-
Single and population coding of taste in the gustatory cortex of awake mice.J Neurophysiol. 2019 Oct 1;122(4):1342-1356. doi: 10.1152/jn.00357.2019. Epub 2019 Jul 24. J Neurophysiol. 2019. PMID: 31339800 Free PMC article.
-
Local field potentials in the gustatory cortex carry taste information.J Neurosci. 2014 Jun 25;34(26):8778-87. doi: 10.1523/JNEUROSCI.0908-14.2014. J Neurosci. 2014. PMID: 24966378 Free PMC article.
-
Statistical analysis and decoding of neural activity in the rodent geniculate ganglion using a metric-based inference system.PLoS One. 2013 May 30;8(5):e65439. doi: 10.1371/journal.pone.0065439. Print 2013. PLoS One. 2013. PMID: 23738016 Free PMC article.
-
The neural processing of taste.BMC Neurosci. 2007 Sep 18;8 Suppl 3(Suppl 3):S5. doi: 10.1186/1471-2202-8-S3-S5. BMC Neurosci. 2007. PMID: 17903281 Free PMC article. Review.
-
The taste of sugars.Neurosci Biobehav Rev. 2008 Jul;32(5):1024-43. doi: 10.1016/j.neubiorev.2008.04.002. Epub 2008 Apr 18. Neurosci Biobehav Rev. 2008. PMID: 18499254 Free PMC article. Review.
References
-
- Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJP, Zuker CS (2000). A novel family of mammalian taste receptors. Cell 100:693–702. - PubMed
-
- Bradley A, Skottun BC, Ohzawa I, Sclar G, Freeman RD (1987). Visual orientation and spatial frequency discrimination: a comparison of single neurons and behavior. J Neurophysiol 57:755–772. - PubMed
-
- Cheng Z, Wasserman GS (1996). Receiver operating characteristic (ROC) analysis of neural code efficacies. II. Optic nerve action potentials and neural transmission. Biol Cybern 75:105–115. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
