Multisensory activation of the intraparietal area when classifying grating orientation: a functional magnetic resonance imaging study
- PMID: 16837597
- PMCID: PMC6674180
- DOI: 10.1523/JNEUROSCI.0822-06.2006
Multisensory activation of the intraparietal area when classifying grating orientation: a functional magnetic resonance imaging study
Abstract
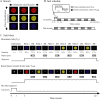
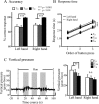
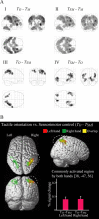
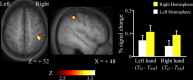
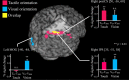
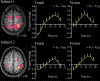
Humans can judge grating orientation by touch. Previous studies indicate that the extrastriate cortex is involved in tactile orientation judgments, suggesting that this area is related to visual imagery. However, it has been unclear which neural mechanisms are crucial for the tactile processing of orientation, because visual imagery is not always required for tactile spatial tasks. We expect that such neural mechanisms involve multisensory areas, because our perception of space is highly integrated across modalities. The current study uses functional magnetic resonance imaging during the classification of grating orientations to evaluate the neural substrates responsible for the multisensory spatial processing of orientation. We hypothesized that a region within the intraparietal sulcus (IPS) would be engaged in orientation processing, regardless of the sensory modality. Sixteen human subjects classified the orientations of passively touched gratings and performed two control tasks with both the right and left hands. Tactile orientation classification activated regions around the right postcentral sulcus and IPS, regardless of the hand used, when contrasted with roughness classification of the same stimuli. Right-lateralized activation was confirmed in these regions by evaluating the hemispheric effects of tactile spatial processing with both hands. In contrast, visual orientation classification activated the left middle occipital gyrus when contrasted with color classification of the same stimuli. Furthermore, visual orientation classification activated a part of the right IPS that was also activated by the tactile orientation task. Thus, we suggest that a part of the right IPS is engaged in the multisensory spatial processing of grating orientation.
Figures

Similar articles
-
Asymmetrical neural substrates of tactile discrimination in humans: a functional magnetic resonance imaging study.J Neurosci. 2004 Aug 25;24(34):7524-30. doi: 10.1523/JNEUROSCI.1395-04.2004. J Neurosci. 2004. PMID: 15329399 Free PMC article.
-
Tactile-visual integration in the posterior parietal cortex: a functional magnetic resonance imaging study.Brain Res Bull. 2008 Mar 28;75(5):513-25. doi: 10.1016/j.brainresbull.2007.09.004. Epub 2007 Oct 8. Brain Res Bull. 2008. PMID: 18355627
-
Tactile form and location processing in the human brain.Proc Natl Acad Sci U S A. 2005 Aug 30;102(35):12601-5. doi: 10.1073/pnas.0505907102. Epub 2005 Aug 22. Proc Natl Acad Sci U S A. 2005. PMID: 16116098 Free PMC article.
-
Hierarchical somatosensory processing.Curr Opin Neurobiol. 1998 Aug;8(4):522-8. doi: 10.1016/s0959-4388(98)80041-x. Curr Opin Neurobiol. 1998. PMID: 9751655 Review.
-
Bilateral Intraparietal Activation for Number Tasks in Studies Using Adaptation Paradigm: A Meta-analysis.Neuroscience. 2022 May 10;490:296-308. doi: 10.1016/j.neuroscience.2022.02.024. Epub 2022 Mar 9. Neuroscience. 2022. PMID: 35276305 Review.
Cited by
-
Tactile priming modulates the activation of the fronto-parietal circuit during tactile angle match and non-match processing: an fMRI study.Front Hum Neurosci. 2014 Dec 15;8:926. doi: 10.3389/fnhum.2014.00926. eCollection 2014. Front Hum Neurosci. 2014. PMID: 25566010 Free PMC article.
-
Visual topography of human intraparietal sulcus.J Neurosci. 2007 May 16;27(20):5326-37. doi: 10.1523/JNEUROSCI.0991-07.2007. J Neurosci. 2007. PMID: 17507555 Free PMC article.
-
Prominent activation of the intraparietal and somatosensory areas during angle discrimination by intra-active touch.Hum Brain Mapp. 2012 Dec;33(12):2957-70. doi: 10.1002/hbm.21419. Epub 2011 Oct 22. Hum Brain Mapp. 2012. PMID: 22020967 Free PMC article.
-
Attention maps in the brain.Wiley Interdiscip Rev Cogn Sci. 2013 Jul;4(4):327-340. doi: 10.1002/wcs.1230. Epub 2013 Feb 27. Wiley Interdiscip Rev Cogn Sci. 2013. PMID: 25089167 Free PMC article. Review.
-
Multisensory integration of sounds and vibrotactile stimuli in processing streams for "what" and "where".J Neurosci. 2009 Sep 2;29(35):10950-60. doi: 10.1523/JNEUROSCI.0910-09.2009. J Neurosci. 2009. PMID: 19726653 Free PMC article.
References
-
- Andersen RA, Snyder LH, Bradley DC, Xing J (1997). Multisensory representation of space in the posterior parietal cortex and its use in planning movements. Annu Rev Neurosci 20:303–330. - PubMed
-
- Bodegard A, Geyer S, Grefkes C, Zilles K, Roland PE (2001). Hierarchical processing of tactile shape in the human brain. Neuron 31:317–328. - PubMed
-
- Bremmer F, Schlack A, Shah NJ, Zafiris O, Kubischik M, Hoffmann K, Zilles K, Fink GR (2001). Polymodal motion processing in posterior parietal and premotor cortex: a human fMRI study strongly implies equivalencies between humans and monkeys. Neuron 29:287–296. - PubMed
-
- Carpenter PA, Eisenberg P (1978). Mental rotation and the frame of reference in blind and sighted individuals. Percept Psychophys 23:117–124. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
