Predifferentiated embryonic stem cells prevent chronic pain behaviors and restore sensory function following spinal cord injury in mice
- PMID: 16838066
- PMCID: PMC1514553
- DOI: 10.2119/2006-00014.Hendricks
Predifferentiated embryonic stem cells prevent chronic pain behaviors and restore sensory function following spinal cord injury in mice
Abstract
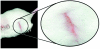
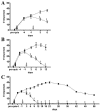
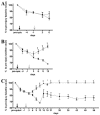
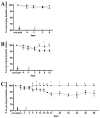
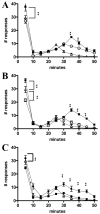
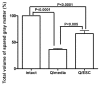
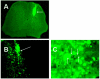
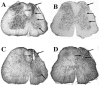
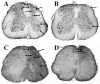
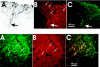
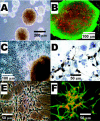
Embryonic stem (ES) cells have been investigated in repair of the CNS following neuronal injury and disease; however, the efficacy of these cells in treatment of postinjury pain is far from clear. In this study, we evaluated the therapeutic potential of predifferentiated mouse ES cells to restore sensory deficits following spinal cord injury (SCI) in mice. The pain model used unilateral intraspinal injection of quisqualic acid (QUIS) into the dorsal horn between vertebral levels T13 and L1. Seven days later, 60,000 predifferentiated ES cells or media were transplanted into the site of the lesion. Histological analysis at 7, 14, and 60 days post-transplantation revealed that animals receiving ES cell transplants suffered significantly less tissue damage than animals receiving media alone. Transplanted cells provided immediate effects on both spontaneous and evoked pain behaviors. Treatment with ES cells resulted in 0% (n = 28) excessive grooming behavior versus 60% (18 of 30) in media-treated animals. In the acetone test (to assess thermal allodynia), mice recovered to preinjury levels by 12 days after ES cell transplant, whereas control animals injected with media after SCI did not show any improvement up to 60 days. Similarly, the von Frey test (to assess mechanical allodynia) and the formalin test (to assess nociceptive hyperalgesia) showed that transplantation of predifferentiated ES cells significantly reduced these pain behaviors following injury. Here we show that predifferentiated ES cells act in a neuroprotective manner and provide antinociceptive and therapeutic effects following excitotoxic SCI.
Figures
References
-
- France RD, Krishnan KRR. (1988) Chronic pain. American Psychiatric Press, Washington, DC, pp. xx, 561.
-
- Guttmann L. (1973) Spinal cord injuries: comprehensive management and research. Blackwell Scientific, Oxford, pp. xiii, 694 p.
-
- Windle WF. (1980) The spinal cord and its reaction to traumatic injury: anatomy-physiology-pharmacology-therapeutics. M. Dekker, New York, pp. xi, 384.
-
- Yezierski RP, Liu S, Ruenes GL, Kajander KJ, Brewer KL. Excitotoxic spinal cord injury: behavioral and morphological characteristics of a central pain model. Pain. 1998;75:141–55. - PubMed
-
- Siddall PJ, Loeser JD. Pain following spinal cord injury. Spinal Cord. 2001;39:63–73. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
