Phytosphingosine stimulates the differentiation of human keratinocytes and inhibits TPA-induced inflammatory epidermal hyperplasia in hairless mouse skin
- PMID: 16838068
- PMCID: PMC1514555
- DOI: 10.2119/2006-00001.Kim
Phytosphingosine stimulates the differentiation of human keratinocytes and inhibits TPA-induced inflammatory epidermal hyperplasia in hairless mouse skin
Abstract
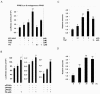
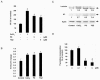
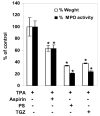
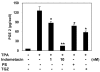
The binding of sphingoid bases to peroxisome proliferator-activated receptor (PPAR) has been detected in a solid-phase binding assay. However, sphingoid base-induced changes in PPAR transactivation activity have not been examined. In this report, we show by reporter gene analyses that phytosphingosine (PS), a natural sphingoid base, activates the transcriptional activity of PPARs in the immortalized human keratinocyte, HaCaT. Real-time PCR analyses showed that the mRNA level of PPARgamma was increased after PS treatment in HaCaT cells in a dose- and time-dependent manner. Because PPARs play important roles in skin barrier homeostasis by regulating epidermal cell growth, terminal differentiation, and inflammatory response, we examined the effect of PS on normal human epidermal keratinocytes (NHEKs) and mouse skin. PS increased the production of cornified envelope in NHEKs by approximately 1.8-fold compared with controls. Epidermal differentiation marker proteins such as involucrin, loricrin, and keratin1 were also increased in PS-treated NHEKs, by ELISA or Western blotting analysis. A [(3)H]thymidine incorporation assay showed that PS inhibited DNA synthesis in NHEKs to 20% compared with controls. The antiproliferative and anti-inflammatory effects of PS were examined in a mouse model of irritant contact dermatitis produced by topical application of 12-O-tetradecanoylphorbol-13-acetate (TPA). PS blocked epidermal thickening and edema and the infiltration of inflammatory cells into the dermis in the skin of TPA-treated hairless mice. The anti-inflammatory effects of PS were confirmed by the observation that PS blocked the TPA-induced generation of prostaglandin E(2) in peripheral mononuclear leukocytes. Taken together, our results provide an insight into the multiple regulatory roles of PS in epidermal homeostasis, and furthermore point to the potential use of PS as a therapeutic agent in the treatment of inflammatory and proliferative cutaneous diseases.
Figures


References
-
- Hanley K, et al. Keratinocyte differentiation is stimulated by activators of the nuclear hormone receptor PPARα. J Invest Dermatol. 1998;110:368–75. - PubMed
-
- Hanley K, et al. Fetal epidermal differentiation and barrier development in vivo is accelerated by nuclear hormone receptor activators. J Invest Dermatol. 1999;113:788–95. - PubMed
-
- Komuves LG, et al. Keratinocyte differentiation in hyperproliferative epidermis: topical application of PPARα activators restores tissue homeostasis. J Invest Dermatol. 2000;115:361–7. - PubMed
-
- Komuves LG, et al. Stimulation of PPARα promotes epidermal keratinocyte differentiation in vivo. J Invest Dermatol. 2000;115:353–60. - PubMed
-
- Kuenzli S, Saurat J-H. Peroxisome proliferator-activated receptors in cutaneous biology. Br J Dermatol. 2003;149:229–36. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
