The effect of nimodipine on calcium homeostasis and pain sensitivity in diabetic rats
- PMID: 16838100
- PMCID: PMC11520761
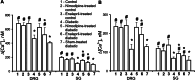
- DOI: 10.1007/s10571-006-9107-z
The effect of nimodipine on calcium homeostasis and pain sensitivity in diabetic rats
Abstract
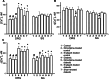
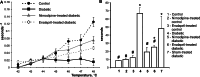
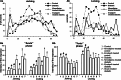
1. The pathogenesis of diabetic neuropathy is a complex phenomenon, the mechanisms of which are not fully understood. Our previous studies have shown that the intracellular calcium signaling is impaired in primary and secondary nociceptive neurons in rats with streptozotocin (STZ)-induced diabetes. Here, we investigated the effect of prolonged treatment with the L-type calcium channel blocker nimodipine on diabetes-induced changes in neuronal calcium signaling and pain sensitivity. 2. Diabetes was induced in young rats (21 p.d.) by a streptozotocin injection. After 3 weeks of diabetes development, the rats were treated with nimodipine for another 3 weeks. The effect of nimodipine treatment on calcium homeostasis in nociceptive dorsal root ganglion neurons (DRG) and substantia gelatinosa (SG) neurons of the spinal cord slices was examined with fluorescent imaging technique. 3. Nimodipine treatment was not able to normalize elevated resting intracellular calcium ([Ca(2+)]( i )) levels in small DRG neurons. However, it was able to restore impaired Ca(2+) release from the ER, induced by either activation of ryanodine receptors or by receptor-independent mechanism in both DRG and SG neurons. 4. The beneficiary effects of nimodipine treatment on [Ca(2+)]( i ) signaling were paralleled with the reversal of diabetes-induced thermal hypoalgesia and normalization of the acute phase of the response to formalin injection. Nimodipine treatment was also able to shorten the duration of the tonic phase of formalin response to the control values. 5. To separate vasodilating effect of nimodipine Biessels et al., (Brain Res. 1035:86-93) from its effect on neuronal Ca(2+) channels, a group of STZ-diabetic rats was treated with vasodilator - enalapril. Enalapril treatment also have some beneficial effect on normalizing Ca(2+) release from the ER, however, it was far less explicit than the normalizing effect of nimodipine. Effect of enalapril treatment on nociceptive behavioral responses was also much less pronounced. It partially reversed diabetes-induced thermal hypoalgesia, but did not change the characteristics of the response to formalin injection. 6. The results of this study suggest that chronic nimodipine treatment may be effective in restoring diabetes-impaired neuronal calcium homeostasis as well as reduction of diabetes-induced thermal hypoalgesia and noxious stimuli responses. The nimodipine effect is mediated through a direct neuronal action combined with some vascular mechanism.
Figures
Similar articles
-
Diabetes-induced changes in calcium homeostasis and the effects of calcium channel blockers in rat and mice nociceptive neurons.Diabetologia. 2001 Oct;44(10):1302-9. doi: 10.1007/s001250100642. Diabetologia. 2001. PMID: 11692179
-
Diabetes-induced abnormalities in ER calcium mobilization in primary and secondary nociceptive neurons.Pflugers Arch. 2004 Jul;448(4):395-401. doi: 10.1007/s00424-004-1263-8. Epub 2004 Mar 27. Pflugers Arch. 2004. PMID: 15048576
-
Failure of nimodipine to prevent or correct the long-term nerve conduction defect and increased neuronal Ca(2+)-currents in the diabetic BB/W-rat.Diabetes Res Clin Pract. 1996 May;32(3):135-40. doi: 10.1016/0168-8227(96)01250-8. Diabetes Res Clin Pract. 1996. PMID: 8858201
-
Cerebrovascular, neuronal, and behavioral effects of long-term Ca2+ channel blockade in aging normotensive and hypertensive rat strains.Ann N Y Acad Sci. 1994 Dec 15;747:431-51. doi: 10.1111/j.1749-6632.1994.tb44427.x. Ann N Y Acad Sci. 1994. PMID: 7847689 Review.
-
Rodent Models of Diabetic Neuropathy, Role of Calcium Homeostasis in Pain and KB-R7943 as a Potential Therapeutic.Int J Mol Sci. 2025 Feb 27;26(5):2094. doi: 10.3390/ijms26052094. Int J Mol Sci. 2025. PMID: 40076715 Free PMC article. Review.
Cited by
-
Abnormal calcium homeostasis in peripheral neuropathies.Cell Calcium. 2010 Feb;47(2):130-9. doi: 10.1016/j.ceca.2009.11.008. Epub 2009 Dec 24. Cell Calcium. 2010. PMID: 20034667 Free PMC article. Review.
-
Insights Into Spinal Dorsal Horn Circuit Function and Dysfunction Using Optical Approaches.Front Neural Circuits. 2020 Jun 12;14:31. doi: 10.3389/fncir.2020.00031. eCollection 2020. Front Neural Circuits. 2020. PMID: 32595458 Free PMC article. Review.
-
Fentanyl Induces Rapid Onset Hyperalgesic Priming: Type I at Peripheral and Type II at Central Nociceptor Terminals.J Neurosci. 2018 Feb 28;38(9):2226-2245. doi: 10.1523/JNEUROSCI.3476-17.2018. Epub 2018 Feb 5. J Neurosci. 2018. PMID: 29431655 Free PMC article.
-
Nociceptive neurons differentially express fast and slow T-type Ca²⁺ currents in different types of diabetic neuropathy.Neural Plast. 2014;2014:938235. doi: 10.1155/2014/938235. Epub 2014 Feb 18. Neural Plast. 2014. PMID: 24693454 Free PMC article.
-
In Vitro Nociceptor Neuroplasticity Associated with In Vivo Opioid-Induced Hyperalgesia.J Neurosci. 2019 Sep 4;39(36):7061-7073. doi: 10.1523/JNEUROSCI.1191-19.2019. Epub 2019 Jul 12. J Neurosci. 2019. PMID: 31300521 Free PMC article.
References
-
- Agrawal, R., Marx, A., and Haller, H. (2006). Efficacy and safety of lercanidipine versus hydrochlorothiazide as add-on to enalapril in diabetic populations with uncontrolled hypertension. J. Hypertens.24:185–192. - PubMed
-
- Biessels, G., and Gispen, W. H. (1996). The calcium hypothesis of brain aging and neurodegenerative disorders: significance in diabetic neuropathy. Life Sci.59:379–387. - PubMed
-
- Biessels, G. J., ter Laak, M. P., Hamers, F. P., and Gispen, W. H. (2002). Neuronal Ca(2+) disregulation in diabetes mellitus. Eur. J. Pharmacol.447:201–209. - PubMed
-
- Biessels, G. J., ter Laak, M. P., Kamal, A., and Gispen, W. H. (2005). Effects of the Ca2+ antagonist nimodipine on functional deficits in the peripheral and central nervous system of streptozotocin-diabetic rats. Brain Res.1035:86–93. - PubMed
-
- Bliss, T. V., and Collingridge, G. L. (1993). A synaptic model of memory: Long-term potentiation in the hippocampus. Nature361:31–39. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

