The neonatal splice variant of Nav1.5 potentiates in vitro invasive behaviour of MDA-MB-231 human breast cancer cells
- PMID: 16838113
- PMCID: PMC4122814
- DOI: 10.1007/s10549-006-9281-1
The neonatal splice variant of Nav1.5 potentiates in vitro invasive behaviour of MDA-MB-231 human breast cancer cells
Abstract
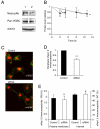
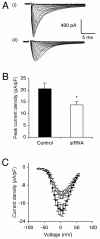
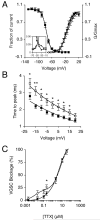
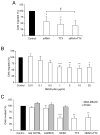
Upregulation of functional voltage-gated Na+ channels (VGSCs) occurs in metastatic human breast cancer (BCa) in vitro and in vivo. The present study aimed to ascertain the specific involvement of the "neonatal" splice variant of Nav1.5 (nNav1.5), thought to be predominant, in the VGSC-dependent invasive behaviour of MDA-MB-231 cells. Functional activity of nNav1.5 was suppressed by two different methods targeting nNav1.5: (i) small interfering RNA (siRNA), and (ii) a polyclonal antibody (NESO-pAb); effects upon migration and invasion were determined. nNav1.5 mRNA, protein and signalling were measured using real-time PCR, Western blotting, and patch clamp recording, respectively. Treatment with the siRNA rapidly reduced (by approximately 90%) the level of nNav1.5 (but not adult Nav1.5) mRNA, but the protein reduction was much smaller (approximately 30%), even after 13 days. Nevertheless, the siRNA reduced peak VGSC current density by 33%, and significantly increased the cells' sensitivity to nanomolar tetrodotoxin (TTX). Importantly, the siRNA suppressed in vitro migration by 43%, and eliminated the normally inhibitory effect of TTX. Migrated MDA-MB-231 cells expressed more nNav1.5 protein at the plasma membrane than non-migrated cells. Furthermore, NESO-pAb reduced migration by up to 42%, in a dose-dependent manner. NESO-pAb also reduced Matrigel invasion without affecting proliferation. TTX had no effect on cells already treated with NESO-pAb. It was concluded that nNav1.5 is primarily responsible for the VGSC-dependent enhancement of invasive behaviour in MDA-MB-231 cells. Accordingly, targeting nNav1.5 expression/activity may be useful in clinical management of metastatic BCa.
Figures


References
-
- Hille B. Ionic channels of excitable membranes. 2nd edn. Sinauer Associates Inc.; Sunderland (Massachusetts): 1992.
-
- Jurkat-Rott K, Lehmann-Horn F. Human muscle voltage-gated ion channels and hereditary disease. Curr Opin Pharmacol. 2001;1(3):280–287. - PubMed
-
- Viswanathan PC, Balser JR. Inherited sodium channelopathies: a continuum of channel dysfunction. Trends Cardiovasc Med. 2004;14(1):28–35. - PubMed
-
- Diss JK, Fraser SP, Djamgoz MB. Voltage-gated Na+ channels: multiplicity of expression, plasticity, functional implications and pathophysiological aspects. Eur Biophys J. 2004;33(3):180–193. - PubMed
-
- Fraser SP, Koyuturk M, Djamgoz MB. Ion channel activity and cancer cell proliferation: A short review with particular reference to prostate cancer. In: Rouzaire-Dubois B, Benoit E, Dubois JM, editors. Ion Channels and Physiopathologies of Nerve Conduction and Cell Proliferation. 1st edn. Research Signpost; 2002.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

