Critical role of Bcr1-dependent adhesins in C. albicans biofilm formation in vitro and in vivo
- PMID: 16839200
- PMCID: PMC1487173
- DOI: 10.1371/journal.ppat.0020063
Critical role of Bcr1-dependent adhesins in C. albicans biofilm formation in vitro and in vivo
Abstract
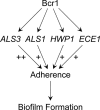
The fungal pathogen Candida albicans is frequently associated with catheter-based infections because of its ability to form resilient biofilms. Prior studies have shown that the transcription factor Bcr1 governs biofilm formation in an in vitro catheter model. However, the mechanistic role of the Bcr1 pathway and its relationship to biofilm formation in vivo are unknown. Our studies of biofilm formation in vitro indicate that the surface protein Als3, a known adhesin, is a key target under Bcr1 control. We show that an als3/als3 mutant is biofilm-defective in vitro, and that ALS3 overexpression rescues the biofilm defect of the bcr1/bcr1 mutant. We extend these findings with an in vivo venous catheter model. The bcr1/bcr1 mutant is unable to populate the catheter surface, though its virulence suggests that it has no growth defect in vivo. ALS3 overexpression rescues the bcr1/bcr1 biofilm defect in vivo, thus arguing that Als3 is a pivotal Bcr1 target in this setting. Surprisingly, the als3/als3 mutant forms a biofilm in vivo, and we suggest that additional Bcr1 targets compensate for the Als3 defect in vivo. Indeed, overexpression of Bcr1 targets ALS1, ECE1, and HWP1 partially restores biofilm formation in a bcr1/bcr1 mutant background in vitro, though these genes are not required for biofilm formation in vitro. Our findings demonstrate that the Bcr1 pathway functions in vivo to promote biofilm formation, and that Als3-mediated adherence is a fundamental property under Bcr1 control. Known adhesins Als1 and Hwp1 also contribute to biofilm formation, as does the novel protein Ece1.
Conflict of interest statement
Figures








References
-
- Douglas LJ. Candida biofilms and their role in infection. Trends Microbiol. 2003;11:30–36. - PubMed
-
- Fux CA, Costerton JW, Stewart PS, Stoodley P. Survival strategies of infectious biofilms. Trends Microbiol. 2005;13:34–40. - PubMed
-
- Kumamoto CA. Candida biofilms. Curr Opin Microbiol. 2002;5:608–611. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

