Nuclear retention of multiply spliced HIV-1 RNA in resting CD4+ T cells
- PMID: 16839202
- PMCID: PMC1487174
- DOI: 10.1371/journal.ppat.0020068
Nuclear retention of multiply spliced HIV-1 RNA in resting CD4+ T cells
Abstract
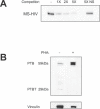
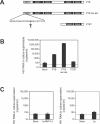
HIV-1 latency in resting CD4+ T cells represents a major barrier to virus eradication in patients on highly active antiretroviral therapy (HAART). We describe here a novel post-transcriptional block in HIV-1 gene expression in resting CD4+ T cells from patients on HAART. This block involves the aberrant localization of multiply spliced (MS) HIV-1 RNAs encoding the critical positive regulators Tat and Rev. Although these RNAs had no previously described export defect, we show that they exhibit strict nuclear localization in resting CD4+ T cells from patients on HAART. Overexpression of the transcriptional activator Tat from non-HIV vectors allowed virus production in these cells. Thus, the nuclear retention of MS HIV-1 RNA interrupts a positive feedback loop and contributes to the non-productive nature of infection of resting CD4+ T cells. To define the mechanism of nuclear retention, proteomic analysis was used to identify proteins that bind MS HIV-1 RNA. Polypyrimidine tract binding protein (PTB) was identified as an HIV-1 RNA-binding protein differentially expressed in resting and activated CD4+ T cells. Overexpression of PTB in resting CD4+ T cells from patients on HAART allowed cytoplasmic accumulation of HIV-1 RNAs. PTB overexpression also induced virus production by resting CD4+ T cells. Virus culture experiments showed that overexpression of PTB in resting CD4+ T cells from patients on HAART allowed release of replication-competent virus, while preserving a resting cellular phenotype. Whether through effects on RNA export or another mechanism, the ability of PTB to reverse latency without inducing cellular activation is a result with therapeutic implications.
Conflict of interest statement
Figures







Similar articles
-
Expression kinetics and subcellular localization of HIV-1 regulatory proteins Nef, Tat and Rev in acutely and chronically infected lymphoid cell lines.Arch Virol. 1994;139(3-4):365-78. doi: 10.1007/BF01310798. Arch Virol. 1994. PMID: 7832642
-
Analysis of human immunodeficiency virus type 1 gene expression in latently infected resting CD4+ T lymphocytes in vivo.J Virol. 2003 Jul;77(13):7383-92. doi: 10.1128/jvi.77.13.7383-7392.2003. J Virol. 2003. PMID: 12805437 Free PMC article.
-
Analysis of human immunodeficiency virus type 1 transcriptional elongation in resting CD4+ T cells in vivo.J Virol. 2004 Sep;78(17):9105-14. doi: 10.1128/JVI.78.17.9105-9114.2004. J Virol. 2004. PMID: 15308706 Free PMC article.
-
Regulation of expression of human immunodeficiency virus.New Biol. 1990 Jan;2(1):20-31. New Biol. 1990. PMID: 2078551 Review.
-
RNA-sequence-mediated gene regulation in HIV-1.Infect Agents Dis. 1994 Apr-Jun;3(2-3):68-76. Infect Agents Dis. 1994. PMID: 7812657 Review.
Cited by
-
Endothelial cell stimulation overcomes restriction and promotes productive and latent HIV-1 infection of resting CD4+ T cells.J Virol. 2013 Sep;87(17):9768-79. doi: 10.1128/JVI.01478-13. Epub 2013 Jul 3. J Virol. 2013. PMID: 23824795 Free PMC article.
-
Shutdown of HIV-1 Transcription in T Cells by Nullbasic, a Mutant Tat Protein.mBio. 2016 Jul 5;7(4):e00518-16. doi: 10.1128/mBio.00518-16. mBio. 2016. PMID: 27381288 Free PMC article.
-
ORF57 overcomes the detrimental sequence bias of Kaposi's sarcoma-associated herpesvirus lytic genes.J Virol. 2015 May;89(9):5097-109. doi: 10.1128/JVI.03264-14. Epub 2015 Feb 18. J Virol. 2015. PMID: 25694606 Free PMC article.
-
Neisseria gonorrhoeae enhances HIV-1 infection of primary resting CD4+ T cells through TLR2 activation.J Immunol. 2010 Mar 15;184(6):2814-24. doi: 10.4049/jimmunol.0902125. Epub 2010 Feb 10. J Immunol. 2010. PMID: 20147631 Free PMC article.
-
Between a shock and a hard place: challenges and developments in HIV latency reversal.Curr Opin Virol. 2019 Oct;38:1-9. doi: 10.1016/j.coviro.2019.03.004. Epub 2019 Apr 29. Curr Opin Virol. 2019. PMID: 31048093 Free PMC article. Review.
References
-
- Gulick RM, Mellors JW, Havlir D, Eron JJ, Gonzalez C, et al. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy [see comments] N Engl J Med. 1997;337:734–739. - PubMed
-
- Hammer SM, Squires KE, Hughes MD, Grimes JM, Demeter LM, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med. 1997;337:725–733. - PubMed
-
- Perelson AS, Essunger P, Cao Y, Vesanen M, Hurley A, et al. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature. 1997;387:188–191. - PubMed
-
- Chun TW, Finzi D, Margolick J, Chadwick K, Schwartz D, et al. In vivo fate of HIV-1-infected T cells: Quantitative analysis of the transition to stable latency. Nat Med. 1995;1:1284–1290. - PubMed
-
- Chun TW, Carruth L, Finzi D, Shen X, Digiuseppe JA, et al. Quantitation of latent tissue reservoirs and total body load in HIV-1 infection. Nature. 1997;387:183–188. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

