Methylation of aquaporins in plant plasma membrane
- PMID: 16839310
- PMCID: PMC1635436
- DOI: 10.1042/BJ20060569
Methylation of aquaporins in plant plasma membrane
Abstract
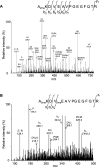
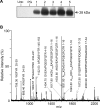
A thorough analysis, using MS, of aquaporins expressed in plant root PM (plasma membrane) was performed, with the objective of revealing novel post-translational regulations. Here we show that the N-terminal tail of PIP (PM intrinsic protein) aquaporins can exhibit multiple modifications and is differentially processed between members of the PIP1 and PIP2 subclasses. Thus the initiating methionine was acetylated or cleaved in native PIP1 and PIP2 isoforms respectively. In addition, several residues were detected to be methylated in PIP2 aquaporins. Lys3 and Glu6 of PIP2;1, one of the most abundant aquaporins in the PM, occurred as di- and mono-methylated residues respectively. Ectopic expression in Arabidopsis suspension cells of PIP2;1, either wild-type or with altered methylation sites, revealed an interplay between methylation at the two sites. Measurements of water transport in PM vesicles purified from these cells suggested that PIP2;1 methylation does not interfere with the aquaporin intrinsic water permeability. In conclusion, the present study identifies methylation as a novel post-translational modification of aquaporins, and even plant membrane proteins, and may represent a critical advance towards the identification of new regulatory mechanisms of membrane transport.
Figures


Similar articles
-
A proteomic study reveals novel insights into the diversity of aquaporin forms expressed in the plasma membrane of plant roots.Biochem J. 2003 Jul 1;373(Pt 1):289-96. doi: 10.1042/BJ20030159. Biochem J. 2003. PMID: 12678916 Free PMC article.
-
Analysis of root plasma membrane aquaporins from Brassica oleracea: post-translational modifications, de novo sequencing and detection of isoforms by high resolution mass spectrometry.J Proteome Res. 2010 Jul 2;9(7):3479-94. doi: 10.1021/pr901150g. J Proteome Res. 2010. PMID: 20462273
-
Role of a single aquaporin isoform in root water uptake.Plant Cell. 2003 Feb;15(2):509-22. doi: 10.1105/tpc.008888. Plant Cell. 2003. PMID: 12566588 Free PMC article.
-
Plant aquaporins: novel functions and regulation properties.FEBS Lett. 2007 May 25;581(12):2227-36. doi: 10.1016/j.febslet.2007.03.021. Epub 2007 Mar 15. FEBS Lett. 2007. PMID: 17382935 Review.
-
PIP1 aquaporins: Intrinsic water channels or PIP2 aquaporin modulators?FEBS Lett. 2015 Nov 30;589(23):3508-15. doi: 10.1016/j.febslet.2015.10.018. Epub 2015 Oct 23. FEBS Lett. 2015. PMID: 26526614 Review.
Cited by
-
Aquaporins are main contributors to root hydraulic conductivity in pearl millet [Pennisetum glaucum (L) R. Br.].PLoS One. 2020 Oct 1;15(10):e0233481. doi: 10.1371/journal.pone.0233481. eCollection 2020. PLoS One. 2020. PMID: 33001997 Free PMC article.
-
A New Set of Golden-Gate-Based Organelle Marker Plasmids for Colocalization Studies in Plants.Plants (Basel). 2022 Oct 5;11(19):2620. doi: 10.3390/plants11192620. Plants (Basel). 2022. PMID: 36235483 Free PMC article.
-
Plasma membrane aquaporins of the PIP1 and PIP2 subfamilies facilitate hydrogen peroxide diffusion into plant roots.BMC Plant Biol. 2022 Dec 5;22(1):566. doi: 10.1186/s12870-022-03962-6. BMC Plant Biol. 2022. PMID: 36471241 Free PMC article.
-
Coordinated post-translational responses of aquaporins to abiotic and nutritional stimuli in Arabidopsis roots.Mol Cell Proteomics. 2013 Dec;12(12):3886-97. doi: 10.1074/mcp.M113.028241. Epub 2013 Sep 20. Mol Cell Proteomics. 2013. PMID: 24056735 Free PMC article.
-
Transcriptome dynamics along with expression analysis of key genes involved in fiber development between Gossypium barbadense and Gossypium darwinii.BMC Plant Biol. 2025 May 23;25(1):691. doi: 10.1186/s12870-025-06697-2. BMC Plant Biol. 2025. PMID: 40410746 Free PMC article.
References
-
- Zardoya R., Villalba S. A phylogenetic framework for the aquaporin family in eukaryotes. J. Mol. Evol. 2001;52:391–404. - PubMed
-
- Luu D.-T., Maurel C. Aquaporins in a challenging environment: molecular gears for adjusting plant water status. Plant Cell Environ. 2005;28:85–96.
-
- Tornroth-Horsefield S., Wang Y., Hedfalk K., Johanson U., Karlsson M., Tajkhorshid E., Neutze R., Kjellbom P. Structural mechanism of plant aquaporin gating. Nature. 2006;439:688–694. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

