Reduction of human T-cell leukemia virus type 1 (HTLV-1) proviral loads in rats orally infected with HTLV-1 by reimmunization with HTLV-1-infected cells
- PMID: 16840318
- PMCID: PMC1563733
- DOI: 10.1128/JVI.00230-06
Reduction of human T-cell leukemia virus type 1 (HTLV-1) proviral loads in rats orally infected with HTLV-1 by reimmunization with HTLV-1-infected cells
Abstract
Human T-cell leukemia virus type 1 (HTLV-1) persistently infects humans, and the proviral loads that persist in vivo vary widely among individuals. Elevation in the proviral load is associated with serious HTLV-1-mediated diseases, such as adult T-cell leukemia and HTLV-1-associated myelopathy/tropical spastic paraparesis. However, it remains controversial whether HTLV-1-specific T-cell immunity can control HTLV-1 in vivo. We previously reported that orally HTLV-1-infected rats showed insufficient HTLV-1-specific T-cell immunity that coincided with elevated levels of the HTLV-1 proviral load. In the present study, we found that individual HTLV-1 proviral loads established in low-responding hosts could be reduced by the restoration of HTLV-1-specific T-cell responses. Despite the T-cell unresponsiveness for HTLV-1 in orally infected rats, an allogeneic mixed lymphocyte reaction in the splenocytes and a contact hypersensitivity response in the skin of these rats were comparable with those of naive rats. HTLV-1-specific T-cell response in orally HTLV-1-infected rats could be restored by subcutaneous reimmunization with mitomycin C (MMC)-treated syngeneic HTLV-1-transformed cells. The reimmunized rats exhibited lower proviral loads than untreated orally infected rats. We also confirmed that the proviral loads in orally infected rats decreased after reimmunization in the same hosts. Similar T-cell immune conversion could be reproduced in orally HTLV-1-infected rats by subcutaneous inoculation with MMC-treated primary T cells from syngeneic orally HTLV-1-infected rats. The present results indicate that, although HTLV-1-specific T-cell unresponsiveness is an underlying risk factor for the propagation of HTLV-1-infected cells in vivo, the risk may potentially be reduced by reimmunization, for which autologous HTLV-1-infected cells are a candidate immunogen.
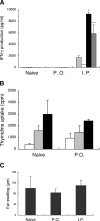
Figures



Similar articles
-
Expansion of human T-cell leukemia virus type 1 (HTLV-1) reservoir in orally infected rats: inverse correlation with HTLV-1-specific cellular immune response.J Virol. 2003 Mar;77(5):2956-63. doi: 10.1128/jvi.77.5.2956-2963.2003. J Virol. 2003. PMID: 12584320 Free PMC article.
-
Virus-host immune interaction in asymptomatic HTLV-1 carriers.Microbiol Spectr. 2025 Mar 4;13(3):e0250724. doi: 10.1128/spectrum.02507-24. Epub 2025 Jan 31. Microbiol Spectr. 2025. PMID: 39887247 Free PMC article.
-
Oral administration of human T-cell leukemia virus type 1 induces immune unresponsiveness with persistent infection in adult rats.J Virol. 1998 Sep;72(9):7289-93. doi: 10.1128/JVI.72.9.7289-7293.1998. J Virol. 1998. PMID: 9696824 Free PMC article.
-
[Anti-tumor immunity in adult T-cell leukemia].Uirusu. 2004 Jun;54(1):67-74. doi: 10.2222/jsv.54.67. Uirusu. 2004. PMID: 15449906 Review. Japanese.
-
Double control systems for human T-cell leukemia virus type 1 by innate and acquired immunity.Cancer Sci. 2011 Apr;102(4):670-6. doi: 10.1111/j.1349-7006.2011.01862.x. Epub 2011 Feb 28. Cancer Sci. 2011. PMID: 21219540 Review.
Cited by
-
Impaired Tax-specific T-cell responses with insufficient control of HTLV-1 in a subgroup of individuals at asymptomatic and smoldering stages.Cancer Sci. 2009 Mar;100(3):481-9. doi: 10.1111/j.1349-7006.2008.01054.x. Epub 2008 Dec 16. Cancer Sci. 2009. PMID: 19154412 Free PMC article.
-
Development and validation of a multiplex real-time PCR assay for simultaneous genotyping and human T-lymphotropic virus type 1, 2, and 3 proviral load determination.J Clin Microbiol. 2009 Nov;47(11):3682-91. doi: 10.1128/JCM.00781-09. Epub 2009 Sep 9. J Clin Microbiol. 2009. PMID: 19741085 Free PMC article.
-
Activation and detection of HTLV-I Tax-specific CTLs by epitope expressing single-chain trimers of MHC class I in a rat model.Retrovirology. 2008 Oct 8;5:90. doi: 10.1186/1742-4690-5-90. Retrovirology. 2008. PMID: 18840303 Free PMC article.
-
Short-term cultured autologous peripheral blood mononuclear cells as a potential immunogen to activate Tax-specific CTL response in adult T-cell leukemia patients.Cancer Sci. 2021 Mar;112(3):1161-1172. doi: 10.1111/cas.14800. Epub 2021 Feb 5. Cancer Sci. 2021. PMID: 33410215 Free PMC article.
-
The roles of acquired and innate immunity in human T-cell leukemia virus type 1-mediated diseases.Front Microbiol. 2012 Sep 3;3:323. doi: 10.3389/fmicb.2012.00323. eCollection 2012. Front Microbiol. 2012. PMID: 22969761 Free PMC article.
References
-
- Arnulf, B., M. Thorel, Y. Poirot, R. Tamouza, E. Boulanger, A. Jaccard, E. Oksenhendler, O. Hermine, and C. Pique. 2004. Loss of the ex vivo but not the reinducible CD8+ T-cell response to Tax in human T-cell leukemia virus type 1-infected patients with adult T-cell leukemia/lymphoma. Leukemia 18:126-132. - PubMed
-
- Cavrois, M., A. Gessain, S. Wain-Hobson, and E. Wattel. 1996. Proliferation of HTLV-1 infected circulating cells in vivo in all asymptomatic carriers and patients with TSP/HAM. Oncogene 12:2419-2423. - PubMed
-
- Chen, S., N. Ishii, S. Ine, S. Ikeda, T. Fujimura, L. C. Ndhlovu, P. Soroosh, K. Tada, H. Harigae, J. Kameoka, N. Kasai, T. Sasaki, and K. Sugamura. 2006. Regulatory T cell-like activity of Foxp3+ adult T cell leukemia cells. Int. Immunol. 18:269-277. - PubMed
-
- Gessain, A., F. Barin, J. C. Vernant, O. Gout, L. Maurs, A. Calender, and G. de The. 1985. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet ii:407-410. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

