Versatile reporter systems show that transactivation by human T-cell leukemia virus type 1 Tax occurs independently of chromatin remodeling factor BRG1
- PMID: 16840326
- PMCID: PMC1563696
- DOI: 10.1128/JVI.00130-06
Versatile reporter systems show that transactivation by human T-cell leukemia virus type 1 Tax occurs independently of chromatin remodeling factor BRG1
Abstract
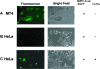
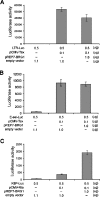
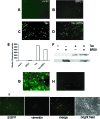
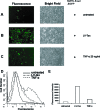
Potent activation of human T-cell leukemia virus type 1 (HTLV-1) gene expression is mediated by the virus-encoded transactivator protein Tax and three imperfect 21-bp repeats in the viral long terminal repeats. Each 21-bp repeat contains a cAMP-responsive-element core flanked by 5' G-rich and 3' C-rich sequences. Tax alone does not bind DNA. Rather, it interacts with basic domain-leucine zipper transcription factors CREB and ATF-1 to form ternary complexes with the 21-bp repeats. In the context of the ternary complexes, Tax contacts the G/C-rich sequences and recruits transcriptional coactivators CREB-binding protein (CBP)/p300 to effect potent transcriptional activation. Using an easily transduced and chromosomally integrated reporter system derived from a self-inactivating lentivirus vector, we showed in a BRG1- and BRM1-deficient adrenal carcinoma cell line, SW-13, that Tax- and 21-bp repeat-mediated transactivation does not require BRG1 or BRM1 and is not enhanced by BRG1. With a similar reporter system, we further demonstrated that Tax- and tumor necrosis factor alpha-induced NF-kappaB activation occurs readily in SW-13 cells in the absence of BRG1 and BRM1. These results suggest that the assembly of stable multiprotein complexes containing Tax, CREB/ATF-1, and CBP/p300 on the 21-bp repeats is the principal mechanism employed by Tax to preclude nucleosome formation at the HTLV-1 enhancer/promoter. This most likely bypasses the need for BRG1-containing chromatin-remodeling complexes. Likewise, recruitment of CBP/p300 by NF-kappaB may be sufficient to disrupt histone-DNA interaction for the initiation of transcription.
Figures


Similar articles
-
HTLV-1 21 bp repeat sequences facilitate stable association between Tax and CREB to increase CREB binding affinity.J Mol Biol. 1996 Nov 22;264(1):20-31. doi: 10.1006/jmbi.1996.0620. J Mol Biol. 1996. PMID: 8950264
-
Acetylation of nucleosomal histones by p300 facilitates transcription from tax-responsive human T-cell leukemia virus type 1 chromatin template.Mol Cell Biol. 2002 Jul;22(13):4450-62. doi: 10.1128/MCB.22.13.4450-4462.2002. Mol Cell Biol. 2002. PMID: 12052856 Free PMC article.
-
Tax abolishes histone H1 repression of p300 acetyltransferase activity at the human T-cell leukemia virus type 1 promoter.J Virol. 2006 Nov;80(21):10542-53. doi: 10.1128/JVI.00631-06. Epub 2006 Aug 30. J Virol. 2006. PMID: 16943293 Free PMC article.
-
Transcriptional and post-transcriptional gene regulation of HTLV-1.Oncogene. 2005 Sep 5;24(39):5938-51. doi: 10.1038/sj.onc.1208973. Oncogene. 2005. PMID: 16155601 Review.
-
The HTLV-1 Tax protein: revealing mechanisms of transcriptional activation through histone acetylation and nucleosome disassembly.Biochim Biophys Acta. 2010 Mar-Apr;1799(3-4):266-74. doi: 10.1016/j.bbagrm.2009.09.002. Epub 2009 Sep 25. Biochim Biophys Acta. 2010. PMID: 19782779 Review.
Cited by
-
Human T-cell leukemia virus type 1 infection leads to arrest in the G1 phase of the cell cycle.J Virol. 2008 Sep;82(17):8442-55. doi: 10.1128/JVI.00091-08. Epub 2008 Jul 2. J Virol. 2008. PMID: 18596104 Free PMC article.
-
A Novel Tax-Responsive Reporter T-Cell Line to Analyze Infection of HTLV-1.Pathogens. 2024 Nov 19;13(11):1015. doi: 10.3390/pathogens13111015. Pathogens. 2024. PMID: 39599568 Free PMC article.
-
KSHV vCyclin counters the senescence/G1 arrest response triggered by NF-κB hyperactivation.Oncogene. 2015 Jan 22;34(4):496-505. doi: 10.1038/onc.2013.567. Epub 2014 Jan 27. Oncogene. 2015. PMID: 24469036 Free PMC article.
-
Dynamics and consequences of the HTLV-1 proviral plus-strand burst.PLoS Pathog. 2022 Nov 28;18(11):e1010774. doi: 10.1371/journal.ppat.1010774. eCollection 2022 Nov. PLoS Pathog. 2022. PMID: 36441826 Free PMC article.
-
Epigenetic Regulation of Human T-Cell Leukemia Virus Gene Expression.Microorganisms. 2021 Dec 31;10(1):84. doi: 10.3390/microorganisms10010084. Microorganisms. 2021. PMID: 35056532 Free PMC article. Review.
References
-
- Baranger, A. M., C. R. Palmer, M. K. Hamm, H. A. Giebler, A. Brauweiler, J. K. Nyborg, and A. Schepartz. 1995. Mechanism of DNA-binding enhancement by the human T-cell leukaemia virus transactivator Tax. Nature 376:606-608. - PubMed
-
- Bauer, W. R., J. J. Hayes, J. H. White, and A. P. Wolffe. 1994. Nucleosome structural changes due to acetylation. J. Mol. Biol. 236:685-690. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

