Influenza virus receptor specificity and cell tropism in mouse and human airway epithelial cells
- PMID: 16840327
- PMCID: PMC1563738
- DOI: 10.1128/JVI.02677-05
Influenza virus receptor specificity and cell tropism in mouse and human airway epithelial cells
Abstract
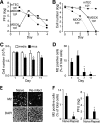
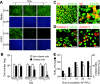
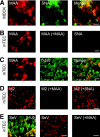
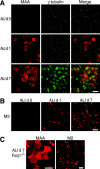
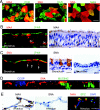
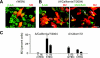
Recent human infections caused by the highly pathogenic avian influenza virus H5N1 strains emphasize an urgent need for assessment of factors that allow viral transmission, replication, and intra-airway spread. Important determinants for virus infection are epithelial cell receptors identified as glycans terminated by an alpha2,3-linked sialic acid (SA) that preferentially bind avian strains and glycans terminated by an alpha2,6-linked SA that bind human strains. The mouse is often used as a model for study of influenza viruses, including recent avian strains; however, the selectivity for infection of specific respiratory cell populations is not well described, and any relationship between receptors in the mouse and human lungs is incompletely understood. Here, using in vitro human and mouse airway epithelial cell models and in vivo mouse infection, we found that the alpha2,3-linked SA receptor was expressed in ciliated airway and type II alveolar epithelial cells and was targeted for cell-specific infection in both species. The alpha2,6-linked SA receptor was not expressed in the mouse, a factor that may contribute to the inability of some human strains to efficiently infect the mouse lung. In human airway epithelial cells, alpha2,6-linked SA was expressed and functional in both ciliated and goblet cells, providing expanded cellular tropism. Differences in receptor and cell-specific expression in these species suggest that differentiated human airway epithelial cell cultures may be superior for evaluation of some human strains, while the mouse can provide a model for studying avian strains that preferentially bind only the alpha2,3-linked SA receptor.
Figures

References
-
- Angata, T., and A. Varki. 2002. Chemical diversity in the sialic acids and related alpha-keto acids: an evolutionary perspective. Chem. Rev. 102:439-469. - PubMed
-
- Baum, L. G., and J. C. Paulson. 1990. Sialyloligosaccharides of the respiratory epithelium in the selection of human influenza virus receptor specificity. Acta Histochem. Suppl. 40:35-38. - PubMed
-
- Beigel, J. H., J. Farrar, A. M. Han, F. G. Hayden, R. Hyer, M. D. de Jong, S. Lochindarat, T. K. Nguyen, T. H. Nguyen, T. H. Tran, A. Nicoll, S. Touch, and K. Y. Yuen. 2005. Avian influenza A (H5N1) infection in humans. N. Engl. J. Med. 353:1374-1385. - PubMed
-
- Bender, C., H. Hall, J. Huang, A. Klimov, N. Cox, A. Hay, V. Gregory, K. Cameron, W. Lim, and K. Subbarao. 1999. Characterization of the surface proteins of influenza A (H5N1) viruses isolated from humans in 1997-1998. Virology 254:115-123. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

