Newcastle disease virus exerts oncolysis by both intrinsic and extrinsic caspase-dependent pathways of cell death
- PMID: 16840332
- PMCID: PMC1563725
- DOI: 10.1128/JVI.00241-06
Newcastle disease virus exerts oncolysis by both intrinsic and extrinsic caspase-dependent pathways of cell death
Abstract
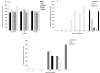
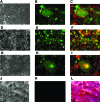
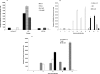
Newcastle disease virus (NDV), an avian paramyxovirus, is tumor selective and intrinsically oncolytic. Here, we present evidence that genetically modified, recombinant NDV strains are cytotoxic to human tumor cell lines of ecto-, endo-, and mesodermal origin. We show that cytotoxicity against tumor cells is due to multiple caspase-dependent pathways of apoptosis independent of interferon signaling competence. The signaling pathways of NDV-induced, cancer cell-selective apoptosis are not well understood. We demonstrate that NDV triggers apoptosis by activating the mitochondrial/intrinsic pathway and that it acts independently of the death receptor/extrinsic pathway. Caspase-8-methylated SH-SY5Y neuroblastoma cells are as sensitive to NDV as other caspase-8-competent cells. This demonstrates that NDV is likely to act primarily through the mitochondrial death pathway. NDV infection results in the loss of mitochondrial membrane potential and the subsequent release of the mitochondrial protein cytochrome c, but the second mitochondrion-derived activator of caspase (Smac/DIABLO) is not released. In addition, we describe early activation of caspase-9 and caspase-3. In contrast, cleavage of caspase-8, which is predominantly activated by the death receptor pathway, is a TNF-related, apoptosis-inducing ligand (TRAIL)-induced late event in NDV-mediated apoptosis of tumor cells. Our data, therefore, indicate that the death signal(s) generated by NDV in tumor cells ultimately converges at the mitochondria and that it acts independently of the death receptor pathway. Our cytotoxicity studies demonstrate that recombinant NDV could be developed as a cancer virotherapy agent, either alone or in combination with therapeutic transgenes. We have also shown that trackable oncolytic NDV could be developed without any reduction in oncolytic efficacy.
Figures


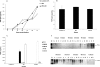

Similar articles
-
Cancer-specific toxicity of apoptin is independent of death receptors but involves the loss of mitochondrial membrane potential and the release of mitochondrial cell-death mediators by a Nur77-dependent pathway.J Cell Sci. 2005 Oct 1;118(Pt 19):4485-93. doi: 10.1242/jcs.02580. J Cell Sci. 2005. PMID: 16179607
-
Tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis of human melanoma is regulated by smac/DIABLO release from mitochondria.Cancer Res. 2001 Oct 1;61(19):7339-48. Cancer Res. 2001. PMID: 11585775
-
TRAIL-induced apoptosis requires Bax-dependent mitochondrial release of Smac/DIABLO.Genes Dev. 2002 Jan 1;16(1):33-45. doi: 10.1101/gad.949602. Genes Dev. 2002. PMID: 11782443 Free PMC article.
-
Newcastle disease virus strain AF2240 as an oncolytic virus: A review.Acta Trop. 2018 Jul;183:126-133. doi: 10.1016/j.actatropica.2018.04.007. Epub 2018 Apr 4. Acta Trop. 2018. PMID: 29626432 Review.
-
Oncolytic Newcastle disease virus for cancer therapy: old challenges and new directions.Future Microbiol. 2012 Mar;7(3):347-67. doi: 10.2217/fmb.12.4. Future Microbiol. 2012. PMID: 22393889 Free PMC article. Review.
Cited by
-
Recombinant Newcastle disease viruses expressing immunological checkpoint inhibitors induce a pro-inflammatory state and enhance tumor-specific immune responses in two murine models of cancer.Front Microbiol. 2024 Jan 24;15:1325558. doi: 10.3389/fmicb.2024.1325558. eCollection 2024. Front Microbiol. 2024. PMID: 38328418 Free PMC article.
-
Histone Deacetylase Inhibitors Enhance Cell Killing and Block Interferon-Beta Synthesis Elicited by Infection with an Oncolytic Parainfluenza Virus.Viruses. 2019 May 10;11(5):431. doi: 10.3390/v11050431. Viruses. 2019. PMID: 31083335 Free PMC article.
-
Combination Therapy of Oncolytic Newcastle Virus and Lenalidomide Enhanced Cytotoxicity in Prostate Cancer Cells.Iran Biomed J. 2025 Jan 1;29(1 & 2):9-19. doi: 10.61186/ibj.4367. Iran Biomed J. 2025. PMID: 40223343 Free PMC article.
-
Oncolysis by paramyxoviruses: preclinical and clinical studies.Mol Ther Oncolytics. 2015;2:15017-. doi: 10.1038/mto.2015.17. Epub 2015 Oct 21. Mol Ther Oncolytics. 2015. PMID: 26640815 Free PMC article.
-
Mesenchymal stem cells enhance the oncolytic effect of Newcastle disease virus in glioma cells and glioma stem cells via the secretion of TRAIL.Stem Cell Res Ther. 2016 Oct 10;7(1):149. doi: 10.1186/s13287-016-0414-0. Stem Cell Res Ther. 2016. PMID: 27724977 Free PMC article.
References
-
- Balasubramanian, A., M. Koziel, J. E. Groopman, and R. K. Ganju. 2005. Molecular mechanism of hepatic injury in coinfection with hepatitis C virus and HIV. Clin. Infect. Dis. 41(Suppl. 1):S32-S37. - PubMed
-
- Bell, J. C., K. A. Garson, B. D. Lichty, and D. F. Stojdl. 2002. Oncolytic viruses: programmable tumour hunters. Curr. Gene Ther. 2:243-254. - PubMed
-
- Bian, H., P. Fournier, B. Peeters, and V. Schirrmacher. 2005. Tumor-targeted gene transfer in vivo via recombinant Newcastle disease virus modified by a bispecific fusion protein. Int. J. Oncol. 27:377-384. - PubMed
-
- Boehning, D., R. L. Patterson, L. Sedaghat, N. O. Glebova, T. Kurosaki, and S. H. Snyder. 2003. Cytochrome c binds to inositol (1,4,5) trisphosphate receptors, amplifying calcium-dependent apoptosis. Nat. Cell Biol. 5:1051-1061. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

